Introduction
Glass Ionomer Cements (GIC) are among the most popular restorative materials, but their use in dentistry is limited due to their physical properties. The hardness of GIC was improved by incorporation of nano-hydroxyapatite-silica into GIC, to expand its applicability.
Aim
To evaluate the cytotoxic effects of nano-hydroxyapatite-silica incorporated glass ionomer cement (HA-SiO2-GIC) on human Dental Pulp Stem Cells (DPSC) and compare it with conventional GIC and resin modified GIC.
Materials and Methods
Material extracts of Fuji IX, Fuji II LC and HA-SiO2-GIC were prepared into seven serial concentrations and applied to 96-well-plates seeded with DPSC. The 96-well-plates were incubated for 24 and 72 hours. The morphology of DPSC was observed under the inverted phase contrast microscope, and the cell viability was determined using MTT assay at both time intervals. Kruskal-Wallis test was performed for statistical analysis.
Results
At maximum concentration, DPSC appeared fewer in number, but the normal spindle morphology was maintained in all groups except for Fuji II LC. At lower concentrations, DPSC appeared normal and more confluent in all groups. The cytotoxic effects of all groups were dose dependent. Fuji IX demonstrated the lowest cytotoxicity, followed by HA-SiO2-GIC. Fuji II LC demonstrated the highest cytotoxicity. The difference was significant between all groups at 200 mg/ml concentration (p<0.05). At concentration <100 mg/ml, cytotoxicity of HA-SiO2-GIC was comparable to that of Fuji IX and lower than that of Fuji II LC.
Conclusion
HA-SiO2-GIC showed a favourable cytotoxicity response and thus holds promise as a future potential restorative material in clinical dentistry.
Introduction
GIC are one of the most popular materials among current dental restoratives [1]. These cements have been successfully applied in dentistry for more than 25 years, ever since their invention [1-4]. These cements are successful because they have unique properties like direct adhesion to the tooth structure and base metals [5], thermal compatibility [6] and low cytotoxicity [7-10]. Even though these Conventional Glass Ionomer Cements (CGIC) have numerous advantages, their use as a restorative material is limited due to brittleness, poor abrasion resistance and lower flexural and tensile strengths [3,6].
While maintaining the advantageous properties of CGIC, Resin-Modified Glass Ionomer Cements (RMGIC) was developed, to overcome the limitations of CGIC. RMGIC demonstrated reduced moisture sensitivity, improved mechanical strengths, extended working time and ease of clinical handling [1,11]. However, they were found to be more cytotoxic than CGIC [9,12,13].
The incorporation of micron range sized particles, such as, alumina, zirconia or glass fibers into CGIC are some efforts made to improve the mechanical strength of CGIC [14-17]. Nevertheless, it did not significantly improve their mechanical strength. These days, the application of nanoscale biomaterials in dentistry is becoming highly popular and favourable. Interestingly, these materials have been reported to exhibit better properties in terms of strength, polishability and aesthetic value as compared to commercial ones [18,19]. Meanwhile, hydroxyapatite is a naturally occurring mineral form of calcium apatite. Synthesis of nanosized hydroxyapatite has now become possible due to various technological advances [20]. As hydroxyapatite has been used as filler in various biomaterials; biocompatibility, hardness analogous to natural tooth structure and intrinsic radiopaque response are some of the benefits that hydroxyapatite (HA) has provided to the field of restorative dentistry [21-24].
There have been many attempts to improve the mechanical properties of CGIC. Improvements in their compressive strength, diametral tensile strength [22], flexural strength [23], toughness, bonding and fluoride-release properties [21] have been reported after the addition of HA into the material. Likewise, the authors have reported a study, wherein the improvement in hardness of commercially available CGIC Fuji IX GP (GC International, Japan) was attempted by incorporating nano-HA-silica powder into it [25,26]. In this case, nano-HA-silica was synthesized locally by one pot technique [25]. Consequently, a 73% increase in hardness of GIC was reported. It is believed that within the GIC matrix, the voids between the hexagonal HA particles are filled in by the nano silica particles. This enhanced the packing density and thus increased its hardness [25,26]. Despite many studies conducted on their physical and mechanical properties, to the best of our knowledge, no studies have been conducted to assess the cytotoxicity or biocompatibility of nano-HA-silica incorporated GIC, particularly on human DPSC. It is well known that the incorporation of resin into GIC has resulted in an increase in its cytoxicity [12,13]. Thus, it may be speculated that the incorporation of nano-HA-silica into GIC and its reaction may result in formation and release of by products or components that are cytotoxic or genotoxic.
Biocompatibility of any dental material should be tested before their use in patients. Animal experiments and cell culture tests are available for testing the biocompatibility of biomaterials or dental materials. While animal experiments are relatively expensive and require extended periods of experimentation, cell culture methods can be performed at lower costs, are relatively faster and easier to perform, and can be reproduced easily [27]. A wide range of in vitro assays have been developed in recent years to evaluate the biocompatibility of various biomaterials. MTT {3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide} is one such in vitro assay, which is a sensitive, quantitative and reliable colorimetric assay that measures viability, proliferation and activation of cells [28]. In living cells, yellow water soluble MTT is reduced to dark blue formazan product by mitochondrial dehydrogenase enzyme [28]. The amount of formazan produced is directly proportional to the number of viable cells present. Hence, measuring the optical density will help in determining the amount of formazan produced and the number of viable cells present, this forms the basis of MTT assay [28].
Restorative materials that are used to replace and restore the loss of tooth structure in the oral cavity normally lie in close proximity to the dental pulp. As such, biocompatibility is one of the important properties that any dental restorative materials should possess. Since dental pulp stem cells play an important role in maintaining a healthy dental pulp [29], substances leaching out from these materials should not be cytotoxic to the pulp tissue, especially to these cells. Hanks CT et al., reported that the reaction of cultured cells to restorative materials is influenced by the type of cells used [30]. Similarly, Huang FM and Chang YC suggested that in vitro cytotoxicity tests should be performed using cells that are homologous to human tissues of ultimate concern [29]. Based on these reasons, the human DPSC were chosen as the cells of interest in this study. Therefore, the purpose of this study were to evaluate the cytotoxic effects of nano-hydroxyapatite-silica incorporated glass ionomer cement (HA-SiO2-GIC) and compare it with the commercially available CGIC and RMGIC, on human DPSC using MTT assay.
Materials and Methods
This was an in vitro comparative experimental study carried out over a period of three months at Craniofacial Science Laboratory, School of Dental Sciences, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia.
A 5% HA-SiO2-GIC was prepared locally as described by Rahman, et al., [25]. Fuji IX GP and capsulated Fuji II LC were purchased from GC Corporation (Japan). Commercially available DPSC were obtained from ALLCELLS (USA). Mesenchymal stem cell (MSC) Basal Medium and supplements were purchased from ALLCELLS (USA). Tryp LE express was purchased from Invitrogen (California, USA). For MTT test, MTT {3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide} powder was obtained from Calbiochem (Darmstadt, Germany), dimethylsulphoxide (DMSO) from Merck (Germany) and ELISA plate reader from Tecan (Switzerland).
Cell culture: Commercially available DPSC were obtained from ALLCELLS (USA) and cultured with MSC Basal Medium + supplements (ALLCELLS, USA). The cells were incubated at 37oC, 5% CO2. Culture medium was changed every 3–4 days until the cells reached confluence. The adherent cells were then detached with TrypLE express (Invitrogen, USA) and the cells were then passaged. The sub cultured cells of 6th passage were used for this experiment.
Preparation of the test material and their extracts: The materials tested were 5% HA-SiO2-GIC, Fuji IX (Fuji IX GP, GC, Japan) and Fuji II LC (Fuji II LC, GC, Japan). A 100 mg of nano-HA-silica powder was added to 1900 mg of Fuji IX powder (Fuji IX GP, GC Japan), to obtain a 5% HA-SiO2-GIC powder mixture. This 5% HA-SiO2-GIC powder mixture was ground manually using a motor and pestle for 10 minutes [25]. The HA-SiO2-GIC cement was then prepared by spatulation and mixing of the 5% HA-SiO2-GIC powder into the Fuji XI liquid (Fuji IX GP, GC Japan) at a powder/liquid ratio of 1:1. The cement was then introduced into a mold to prepare standardized round shaped specimens of 10.0 mm diameter and 2.0 mm height. This mold with HA-SiO2-GIC was then interposed between two glass slides and compressed to obtain flat round specimens [25]. Similarly, round shaped specimens of Fuji IX and Fuji II LC were also prepared following the manufacturer’s instructions. After complete setting, the specimens were removed from the molds and subjected to ultraviolet radiation (Purifier Class II Biosafety Cabinet, Labconco, USA) for 30 minutes to sterilize them. The specimens were then weighed and introduced into sterile glass bottles with MSC Basal Medium + supplements and incubated for 72 hours at 37oC, 5% CO2. The weight to volume ratio was 200 mg/ml, which was set according to the ISO standards [31]. After incubation, the material extracts were passed through a filter (20 μm) into another sterile glass bottle. The extracts were then serially diluted and added to 96 well culture plates seeded with DPSC.
Evaluation of cytotoxicity using MTT assay: The MTT assay was carried out as described in a previous study [32]. DPSC were seeded in 96 well culture plates at 5 x 103 cells per well with 100 μl of MSC Basal Medium + supplements (ALLCELLS, USA) and incubated for 24 hours at 37oC, 5% CO2. The medium was then replaced with 200 μl of fresh medium containing different concentrations of material extracts (200, 100, 50, 25, 12.5, 6.25 and 3.125 mg/ml), prepared by serial dilution. Plain culture medium was used as negative control. Two sets of 96 well culture plates were prepared for each of the three material extracts and each of the two sets were incubated for 24 hours and 72 hours respectively at 37oC, 5% CO2.
After incubation, the morphology of cells in 96 well culture plates were observed under the inverted phase contrast microscope (Carl Zeiss, Germany). The effect of the material extracts on the cell mitochondrial function was measured by MTT assay. A 20 μl of MTT (5 mg/ml) was added into each well to a final concentration of 0.5 mg/ml, and incubated for four hours at 37oC, 5% CO2. Subsequently, the wells were evacuated and 100 μl of Dimethyl Sulphoxide (DMSO) was added to each of the wells. Optical density (OD570) of each well was measured using ELISA plate reader (Sunrise, Tecan, Switzerland) at 570 nm wavelength with reference wavelength of 630 nm. The relative viability of the cells compared to the control was calculated using the following formula.
% Cell Viability= {OD570 of treated cells} × 100%/ {OD570 of control cells} [31].
Statistical Analysis
Data were entered using SPSS Version 2.0 (IBM SPSS, 2013). Kruskal-Wallis test complemented by Mann-Whitney test was used to analyse the data obtained and the level of significance was set at p<0.05.
Results
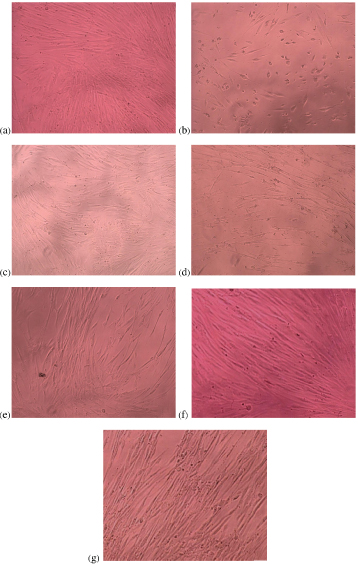
[Table/Fig-1a-g] is a set of photomicrographs showing morphology and density of cells after culturing with material extracts for 72 hours. At highest concentration (200 mg/ml), DPSC appeared fewer in number in all groups. However, normal spindle morphology was lost in cells cultured with highest concentration of Fuji II LC material extract only (200 mg/ml). Cell density increased with decreasing concentration of the material extract for all groups. As for, Fuji II LC, normal cell morphology was maintained at all concentrations<200 mg/ml.
Photomicrographs showing cell morphology and density (200x magnification) after culture with different concentration of extracts for 72 hours: (a) Negative control; (b) 200 mg/ml Fuji II LC extract; (c) 200 mg/ml Fuji IX extract; (d) 200 mg/ml HA-SiO2-GIC extract; (e) 50 mg/ml Fuji II LC extract; (f) 50 mg/ml Fuji IX extract; (g) 50 mg/ml HA-SiO2-GIC extract.
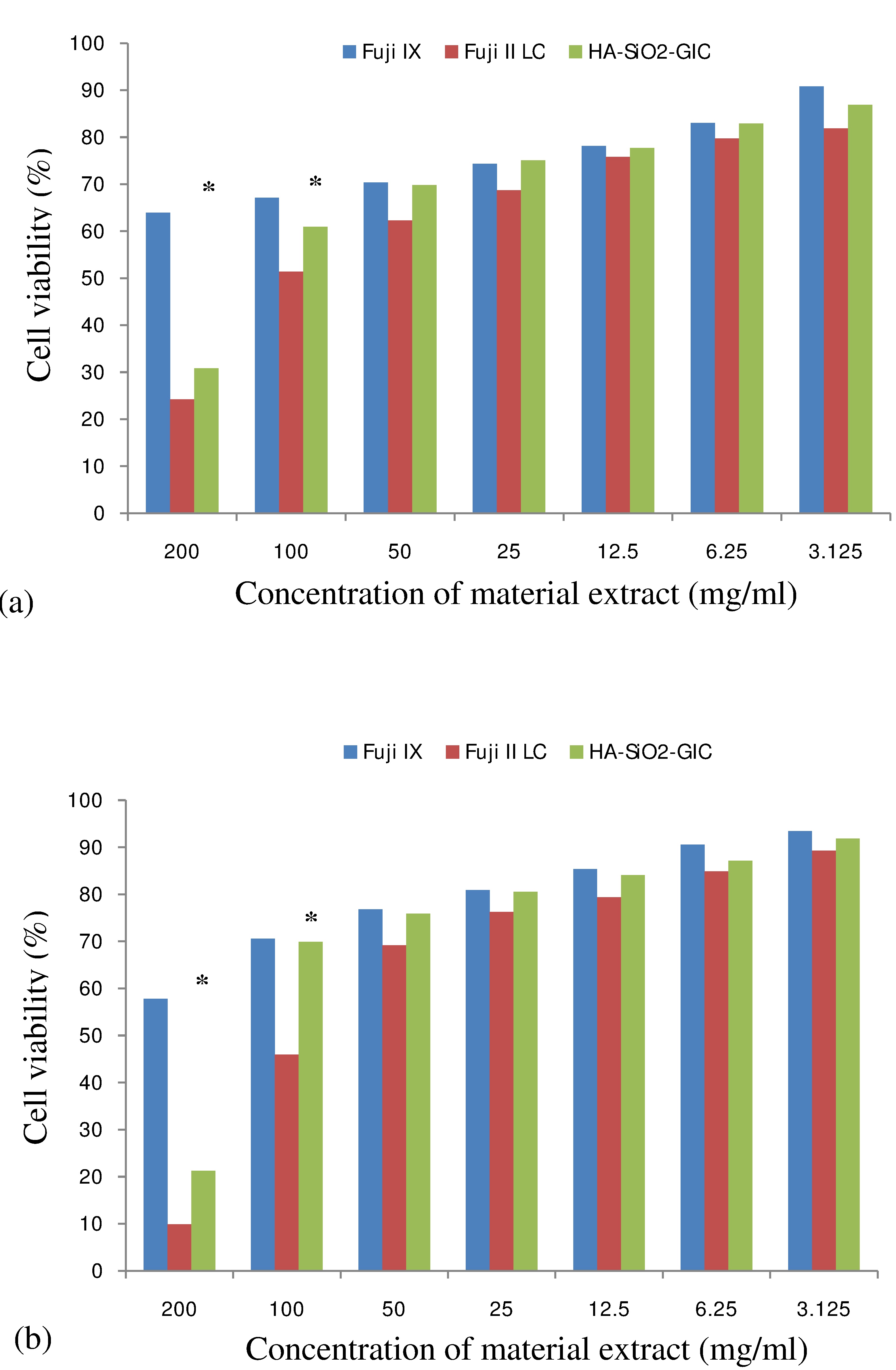
Cell viability (%) determined by MTT assay was found to be the highest with Fuji IX extract at all concentrations after both incubation periods, followed by HA-SiO2-GIC. The least cell viability (%) was found with Fuji II LC extract. The cell viability (%) was significantly different between Fuji IX vs. Fuji II LC and HA-SiO2-GIC vs. Fuji II LC at 200 and 100 mg/ml concentration, after both incubation periods (p<0.05). In the meantime, no statistically significant difference in cell viability (%) was found between HA-SiO2-GIC vs. Fuji IX at any concentration, except at the maximum concentration (200 mg/ml) (p<0.05). At all concentrations < 50 mg/ml, no significant differencein cell viability (%) were found between Fuji IX vs. Fuji II LC and HA-SiO2-GIC vs. Fuji II LC (p>0.05). The results of statistical analysis are presented in [Table/Fig-2,3,4 and 5]. [Table/Fig-6a,b] shows cell viability (%) against material extract concentration after 24 hours and 72 hours incubation period respectively.
Kruskal wallis test results for 24 hours incubation period variable.
| Conc.mg/ml | Material(n=15) | Mean (SD)%24 hrs | Median (IQR)%24 hrs | p-value |
|---|
| 200 | Fuji IX | 63.94 (14.14) | 66.11 (26.44) | < 0.001* |
| Fuji II LC | 24.21 (8.27) | 26.89 (15.89) |
| HA-SiO2-GIC | 30.85 (9.11) | 35.22 (14.57) |
| 100 | Fuji IX | 67.15 (12.11) | 67.31 (25.24) | 0.021* |
| Fuji II LC | 51.43 (12.08) | 55.01 (22.00) |
| HA-SiO2-GIC | 60.97 (19.76) | 68.02 (43.72) |
| 50 | Fuji IX | 70.35 (11.99) | 75.72 (21.63) | 0.147 |
| Fuji II LC | 62.27 (14.27) | 64.79 (6.11) |
| HA-SiO2-GIC | 69.79 (18.41) | 75.30 (32.79) |
| 25 | Fuji IX | 74.36 (12.74) | 79.33 (18.03) | 0.152 |
| Fuji II LC | 68.70 (13.82) | 75.79 (24.45) |
| HA-SiO2-GIC | 75.06 (16.77) | 80.16 (30.36) |
| 12.5 | Fuji IX | 78.13 (11.74) | 80.53 (22.84) | 0.760 |
| Fuji II LC | 75.79 (12.59) | 80.68 (23.23) |
| HA-SiO2-GIC | 77.73 (16.11) | 83.81 (29.15) |
| 6.25 | Fuji IX | 83.01 (16.10) | 91.35 (28.85) | 0.481 |
| Fuji II LC | 79.71 (15.33) | 88.02 (28.12) |
| HA-SiO2-GIC | 82.91 (13.66) | 86.23 (24.29) |
| 3.125 | Fuji IX | 90.79 (15.50) | 96.15 (26.44) | 0.119 |
| Fuji II LC | 81.90 (14.89) | 90.46 (30.56) |
| HA-SiO2-GIC | 86.88 (14.52) | 87.45 (26.72) |
* Statistically significant.
Mann whitney test results for 24 hours incubation period variable.
| Conc.mg/ml | Comparison | Z-statistic | p-value |
|---|
| 200 | Fuji IX / Fuji II LC | -4.670 | < 0.001* |
| Fuji IX / HA-SiO2-GIC | -4.590 | < 0.001* |
| Fuji II LC/ HA-SiO2-GIC | -2.432 | 0.015* |
| 100 | Fuji IX / Fuji II LC | -2.845 | 0.004* |
| Fuji IX / HA-SiO2-GIC | -0.685 | 0.051 |
| Fuji II LC / HA-SiO2-GIC | -1.725 | 0.089 |
* Statistically significant.
Kruskal wallis test results for 72 hours incubation period variable.
| Conc.mg/ml | Material(n=15) | Mean (SD)%72 hrs | Median (IQR)% 72 hrs | p-value |
|---|
| 200 | Fuji IX | 57.83 (11.07) | 63.90 (23.30) | < 0.001* |
| Fuji II LC | 9.86 (2.99) | 11.32 (4.96) |
| HA-SiO2-GIC | 21.27 (7.16) | 25.04 (11.99) |
| 100 | Fuji IX | 70.58 (9.88) | 76.25 (16.95) | < 0.001* |
| Fuji II LC | 45.92 (5.89) | 48.84 (7.79) |
| HA-SiO2-GIC | 69.91 (17.12) | 80.39 (33.85) |
| 50 | Fuji IX | 76.79 (13.81) | 85.08 (26.48) | 0.243 |
| Fuji II LC | 69.18 (10.02) | 75.04 (19.11) |
| HA-SiO2-GIC | 75.91 (15.53) | 84.98 (31.73) |
| 25 | Fuji IX | 80.89 (15.16) | 90.02 (30.71) | 0.665 |
| Fuji II LC | 76.29 (13.69) | 84.24 (26.90) |
| HA-SiO2-GIC | 80.51 (17.21) | 89.92 (34.56) |
| 12.5 | Fuji IX | 85.36 (16.82) | 94.96 (33.89) | 0.567 |
| Fuji II LC | 79.38 (15.46) | 89.19 (29.38) |
| HA-SiO2-GIC | 84.11 (16.03) | 92.74 (29.97) |
| Fuji IX | 90.56 (15.49) | 99.20 (31.07) |
| 6.25 | Fuji II LC | 84.85 (16.09) | 94.15 (32.92) | 0.609 |
| HA-SiO2-GIC | 87.14 (15.56) | 95.91 (26.80 |
| 3.125 | Fuji IX | 93.41 (12.99) | 100.61 (25.06) | 0.095 |
| Fuji II LC | 89.26 (13.86) | 97.33 (23.71) |
| HA-SiO2-GIC | 91.84 (12.16) | 98.38 (19.04) |
* Statistically significant.
Mann whitney test results for 72 hours incubation period variable.
| Conc.mg/ml | Comparison | Z-statistic | p-value |
|---|
| 200 | Fuji IX / Fuji II LC | -4.670 | < 0.001* |
| Fuji IX/ HA-SiO2-GIC | -4.670 | < 0.001* |
| Fuji II LC/ HA-SiO2-GIC | -3.246 | < 0.001* |
| 100 | Fuji IX / Fuji II LC | -4.420 | < 0.001* |
| Fuji IX / HA-SiO2-GIC | -1.474 | 0.141 |
| Fuji II LC / HA-SiO2-GIC | -2.803 | 0.005* |
* Statistically significant.
Cell viability (%) vs. material extract concentration (mg/ml). Cell viability values obtained after incubation with various concentrations of material extracts, statictical analysis carried out using Kruskal Wallis test: (a) 24 hours incubation; (b) 72 hours incubation
* Statistically significant
Discussion
In vitro cytotoxicity tests have been used widely to evaluate the initial biocompatibility of dental materials. In this study, DPSC were used to evaluate the in vitro cytotoxicity of locally produced HA-SiO2-GIC and compare it with the in vitro cytotoxicity of commercially available products, Fuji IX (CGIC) and Fuji II LC (RMGIC) using MTT assay. Additionally, cells were observed under the inverted phase contrast microscope to observe changes in the morphology and density of cells following exposure to material extracts. The cells exposed to 200 mg/ml concentration of Fuji II LC extract demonstrated the lowest cell density with loss of normal spindle morphology as well. Similar changes in morphology following exposure to extracts of Fuji II LC have also been reported previously by other author [33]. Maximum concentration (200 mg/ml) of Fuji IX and HA-SiO2-GIC extract also reduced the cell density. However, their normal spindle morphology was maintained. The changes in morphology together with reduction in cell density indicate that the cytotoxic effects were most significant with extracts of Fuji II LC. This is possibly due to the release of substantial amounts of residual unset monomers such as HEMA from Fuji II LC into the culture medium. Studies found that substantial amounts of HEMA were released from various commercially available RMGIC into the liquid medium, when studied using liquid chromatography [34,35]. The current study also found that the density of cells increased with decreasing concentration of all the material extracts. The cell morphology and density were similar after both incubation periods, suggesting that cells behave in a similar manner when exposed to material extracts for both short and long incubation periods.
The results of the cell viability determined by using MTT assay were consistent with the observation noted when the cells were observed under the inverted phase contrast microscope. The cell viability was found to be the lowest in the culture wells with 200 mg/ml concentration of Fuji II LC extract. This is possibly due to the release of residual monomers such as HEMA from Fuji II LC, as stated earlier. Another study that tested the cytotoxicity of Fuji II LC showed that the cytotoxicity of Fuji II LC reduced considerably when monomers such as HEMA were removed from Fuji II LC using ethanol elution [12]. As such, Stanislawski L et al., [12] concluded that residual monomers released from Fuji II LC is the main cause of its cytotoxicity. The cytotoxic effects of RMGIC have been confirmed with similar findings by other previous studies too, but using various cell lines [9,33,35,36].
Fuji IX and HA-SiO2-GIC also demonstrated some degree of reduction in cell viability. However, the cell viability was more than 50%, even at the maximum concentration (200 mg/ml) for Fuji IX after both 24 hours (63.94+14.14) and 72 hours (57.83+11.07) incubation period. This indicates that Fuji IX (CGIC) exhibited a moderate to low cytotoxicity even at the highest concentration allowed by ISO standards [37]. Similar findings have also been reported previously by other authors [9,32,33]. Additionally, the cell viability of Fuji IX also increased with decreasing concentration of the material extract. The cell viability for Fuji IX was over 90% at lowest concentration (3.125 mg/ml) after 24 hours and 72 hours incubation period, indicating that it was non-cytotoxic at this concentration. All these findings suggest that the components released from Fuji IX are non toxic or only slightly toxic to the cells.
Theoretically, since it does not contain monomers, HA-SiO2-GIC group should not be cytotoxic or should only show mild cytotoxicity like CGIC. However, HA-SiO2-GIC demonstrated a moderate to high cytotoxicity value at maximum concentration (200 mg/ml). We can speculate that the incorporation of nano-HA-silica into GIC may have resulted in the formation and release of by-products or components that may be toxic to the cells at this concentration (200 mg/ml). Based on a study by Shiekh RA et al., it was demonstrated that there was a presence of a high degree of cross linking of silyl species between the nanosilica and glass particles in the GIC matrix [38]. As a result, lesser and fewer glass particles are available to react with the Polyacrylic Acid (PAA) during the setting of HA-SiO2-GIC, thus causing more un-reacted freely available PAA molecules to be present in the set HA-SiO2-GIC matrix. These freely available PAA molecules may be released from HA-SiO2-GIC into the liquid medium. However, no chemical analysis was undertaken in this study to identify the exact components released from HA-SiO2-GIC into the culture medium. Nevertheless, we speculate that the release of freely available PAA molecules from HA-SiO2-GIC into the liquid medium may be the cause of the cytotoxic effects induced by HA-SiO2-GIC at 200 mg/ml concentration, which can only be confirmed by chemical analysis study in future. Despite that, the cell viability demonstrated by HA-SiO2-GIC was significantly higher than the cell viability demonstrated by Fuji II LC at maximum concentration (200 mg/ml). Thus, it is assumed that the residual monomers released from RMGIC (Fuji II LC) are comparatively more toxic to the cells than any of the components released from HA-SiO2-GIC.
At 100 mg/ml concentration, the cell viability demonstrated by HA-SiO2-GIC was comparable and not significantly different from Fuji IX. In contrast, HA-SiO2-GIC still has significantly higher cell viability compared to Fuji II LC. In addition, no significant difference was noted at all other lower concentration. Thus, at concentrations< 100 mg/ml, HA-SiO2-GIC seems to exhibit favourable and comparable findings similar to CGIC and has demonstrated better results compared to RMGIC. A previous study that tested the cytotoxicity and genotoxicity of nano-HA-silica alone, before incorporation into Fuji IX reported a moderate to low cytotoxicity and no genotoxicity at their highest concentration (100 mg/ml) [31]. Similarly, a moderate to low cytotoxicity value of HA-SiO2-GIC was also demonstrated at 100 mg/ml concentration in the current study. Thus, our findings correlate with the findings of a previous study that tested the cytotoxicity and genotoxicity of nano-HA-silica alone [31].
The current study indicates that the cytotoxicity of the materials tested was dose dependent. Similar findings have been reported previously for CGIC and RMGIC [12,33]. As mentioned earlier, to the best of our knowledge, no previous study has reported the cytotoxicity or biocompatibility of nano-HA-silica incorporated GIC.
Limitation
The results of this in vitro cytotoxicity testing should be extrapolated to a clinical situation only with extra caution, as the protective effects of the dentine and the defense and repair mechanisms of the pulp are not considered in this study. Additionally, Commercial primary cells used in this study may not be a representative of the cells derived from the patient and their behaviour may change during passaging.
Conclusion
Fuji IX was found to be the most biocompatible material in terms of its cytotoxicity, followed by HA-SiO2-GIC. On the other hand, Fuji II LC demonstrated the least biocompatibility in terms of cytotoxicity. A favourable biological response was demonstrated by HA-SiO2-GIC, which is comparable to that of CGIC. HA-SiO2-GIC may be considered as promising potential restorative material, however, its potential use needs to be validated by further in vitro and in vivo investigations.
* Statistically significant.
* Statistically significant.
* Statistically significant.
* Statistically significant.
[1]. Smith DC, Development of glass-ionomer cement systemsBiomater 1998 19(6):467-78. [Google Scholar]
[2]. Wilson AD, McLean JW, Glass ionomer cements 1988 Chicago, ILQuintessence Publishing Co, Inc:1-10. [Google Scholar]
[3]. Mount GJ, Clinical performance of glass-ionomersBiomater 1998 19(6):573-79. [Google Scholar]
[4]. Davidson CL, Mjor IA, Advances in glass ionomer cements 1999 Chicago, ILQuintessence Publ Co Inc [Google Scholar]
[5]. Hotz P, McLean JW, Sced I, Wilson AD, The bonding of glass ionomer cements to metal and tooth substratesBr Dent J 1977 142:41-47. [Google Scholar]
[6]. Craig RG, Testing of dental materials and biomechanics. In: Craig RG, editorRestorative dental materials 1997 10th edSt. Louis, MOMosby - Year book, Inc:83-107. [Google Scholar]
[7]. Nicholson JW, Braybrook JH, Wasson EA, The biocompatibility of glass-poly(alkenoate) (glass-ionomer) cements: A reviewJ Biomater Sci Polym ed 1991 2(4):277-85. [Google Scholar]
[8]. Hume WR, Mount GJ, In vitro studies on the potential for pulpal cytotoxicity of glass ionomer cementsJ Dent Res 1988 67(6):915-18. [Google Scholar]
[9]. de Souza Costa CA, Hebling J, Garcia-Godoy F, Hanks CT, In vitro cytotoxicity of five glass-ionomer cementsBiomater 2003 24(21):3853-58. [Google Scholar]
[10]. Klotzer WT, Pulp reactions to a glass ionomer cementJ Dent Res 1975 54:678-85. [Google Scholar]
[11]. Wilson AD, Resin-modified glass-ionomer cementsInt J Prosthodont 1990 3(5):425-29. [Google Scholar]
[12]. Stanislawski L, Daniau X, Lautié A, Goldberg M, Factors responsible for pulp cell cytotoxicity induced by resin-modified glass ionomer cementsJ Biomed Mater Res 1999 48(3):277-88. [Google Scholar]
[13]. Leyhausen G, Abtahi M, Karbakhsch M, Sapotnick A, Geurtsen W, Biocompatibility of various light-curing and one conventional glass-ionomer cementBiomater 1998 19(6):559-64. [Google Scholar]
[14]. Kerby RE, Bleiholder RF, Physical properties of stainless-steel and silver-reinforced glass-ionomer cementsJ Dent Res 1991 70(10):1358-61. [Google Scholar]
[15]. Lohbauer U, Frankenberger R, Clare A, Petschelt A, Greil P, Toughening of dental glass ionomer cements with reactive glass fibresBiomater 2004 25(22):5217-25. [Google Scholar]
[16]. Gu YW, Yap AUJ, Cheang P, Khor KA, Effects of incorporation of ha/zro2 into glass ionomer cement (gic)Biomater 2005 26(7):713-20. [Google Scholar]
[17]. Yli-Urpo H, Lassila LV, Narhi T, Vallittu PK, Compressive strength and surface characterization of glass ionomer cements modified by particles of bioactive glassDent Mater 2005 21(3):201-09. [Google Scholar]
[18]. Mitra SB, Wu D, Holmes BN, An application of nanotechnology in advanced dental materialsJ Am Dent Assoc 2003 134(10):1382-90. [Google Scholar]
[19]. Saunders SA, Current practicality of nanotechnology in dentistry. Part 1: Focus on nanocomposite restoratives and biomimeticsClin Cosmet Investig Dent 2009 1:47-61. [Google Scholar]
[20]. Dorozhkin SV, Nanosized and nanocrystalline calcium orthophosphatesActa Biomater 2010 6(3):715-34. [Google Scholar]
[21]. Lucas ME, Arita K, Nishino M, Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cementBiomater 2003 24(21):3787-94. [Google Scholar]
[22]. Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr JA, Rehman I, Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (gic)Acta Biomater 2008 4(2):432-40. [Google Scholar]
[23]. Arita K, Lucas ME, Nishino M, The effect of adding hydroxyapatite on the flexural strength of glass ionomer cementDent Mater J 2003 22(2):126-36. [Google Scholar]
[24]. Arita K, Yamamoto A, Shinonaga Y, Harada K, Abe Y, Nakagawa K, Hydroxyapatite particle characteristics influence the enhancement of the mechanical and chemical properties of conventional restorative glass ionomer cementDent Mater J 2011 30(5):672-83. [Google Scholar]
[25]. Rahman IA, Masudi SM, Luddin N, Shiekh RA, One-pot synthesis of hydroxyapatite-silica nanopowder composite for hardness enhancement of glass ionomer cement (gic)Bull Mater Sci 2014 37(2):213-19. [Google Scholar]
[26]. Luddin N, Incorporation of hydroxyapatite-silica nano-powder for enhancement of glass ionomer cement (gic)J Interdiscipl Med Dent Sci 2015 3(5):e104 [Google Scholar]
[27]. Schmalz G, Schuster U, Nuetzel K, Schweikl H, An in vitro pulp chamber with three-dimensional cell culturesJ Endod 1999 25(1):24-29. [Google Scholar]
[28]. Vega-Avila E, Pugsley MK, An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cellsProc West Pharmacol Soc 2011 54:10-14. [Google Scholar]
[29]. Huang F-M, Chang Y-C, Cytotoxicity of resin-based restorative materials on human pulp cell culturesOral Surg Oral Med Oral Pathol Oral Radiol Endod 2002 94(3):361-65. [Google Scholar]
[30]. Hanks CT, Anderson M, Craig RG, Cytotoxic effects of dental cements on two cell culture systemsJ Oral Pathol 1981 10(2):101-12. [Google Scholar]
[31]. Musa M, Kannan T, Masudi S, Rahman I, Assessment of DNA damage caused by locally produced hydroxyapatite-silica nanocomposite using comet assay on human lung fibroblast cell lineMol Cell Toxicol 2012 8(1):53-60. [Google Scholar]
[32]. Ahmed HM, Omar NS, Luddin N, Saini R, Saini D, Cytotoxicity evaluation of a new fast set highly viscous conventional glass ionomer cement with l929 fibroblast cell lineJ Conserv Dent 2011 14(4):406-08. [Google Scholar]
[33]. Xie D, Yang Y, Zhao J, Park JG, Zhang JT, A novel comonomer-free light-cured glass-ionomer cement for reduced cytotoxicity and enhanced mechanical strengthDent Mater 2007 23(8):994-1003. [Google Scholar]
[34]. Hamid A, Okamoto A, Iwaku M, Hume WR, Component release from light-activated glass ionomer and compomer cementsJ Oral Rehabil 1998 25(2):94-99. [Google Scholar]
[35]. Geurtsen W, Spahl W, Leyhausen G, Residual monomer/additive release and variability in cytotoxicity of light-curing glass-ionomer cements and compomersJ Dent Res 1998 77(12):2012-19. [Google Scholar]
[36]. Souza PPC, Aranha AMF, Hebling J, Giro E, Costa CA, In vitro cytotoxicity and in vivo biocompatibility of contemporary resin-modified glass-ionomer cementsDent Mater 2006 22(9):838-44. [Google Scholar]
[37]. ISO10993-5. Biological evaluation of medical devices, test for in vitro cytotoxicity. International organization for standardization. 3rd ed. Geneva, Switzerland 2009. Pp. 1-34 [Google Scholar]
[38]. Shiekh RA, Ab Rahman I, Masudi SM, Luddin N, Modification of glass ionomer cement by incorporating hydroxyapatite-silica nano-powder composite: Sol-gel synthesis and characterizationCeram Int 2014 40(2):3165-70. [Google Scholar]