One of the enigmas in cell differentiation process is prosoplasia, which is contemplated as forward differentiation. A well-known example of prosoplastic switch is mucous cell prosoplasia, which is the transformation of a simple squamous epithelial cell into mucous secreting cell. Numerous theories have been proposed for histogenesis of this phenomenon, which are comprehensively discussed in the present paper. Oral pathologies like odontogenic cysts and salivary gland tumours show mucous prosoplasia quite often; sometimes leading to diagnostic difficulties. In the present paper, efforts have been made to comprehensively discuss diverse aspects of mucous prosoplasia like histogenesis, theories and diagnostic importance in various oral pathologies.
Introduction
Cellular differentiation is an important event in the development of organism where a cell changes from one type to another. The differentiated cells are usually more specialized in terms of gene expression and phenotypes [1]. Differentiation occurs throughout the life and involves numerous physiological events that lead to formation of complex system of tissues and cells. However, numerous pathologies also show differentiation in the form of metaplasia, anaplasia and prosoplasia. One of the most enigmatic types of cellular differentiation in pathology is prosoplasia [2].
Prosoplasia is the word derived from two ancient Greek words, ‘prósopon’ meaning ‘face’ and ‘plasis’ meaning ‘formation’. It is the differentiation of cells either to a higher or intricate function or to a complex level of organization. Thus, prosoplasia is basically known as development of a new complicated cell function. It is contemplated as a forward differentiation in contrast to anaplasia, which is considered as a retrograde differentiation [3].
A well-known example of this prosoplastic switch is mucous cell prosoplasia, which is the transformation of a simple squamous epithelial cell into mucous secreting cell. Mucous cells are found in the superficial epithelial lining in numbers varying from scattered cells to uninterrupted rows of cells [4]. In the areas of acanthosis, individual mucous cells in stratum spinosum along with the stratum superficiale are seen. Mucous cells have been reported lining the intraepithelial gland like structures in the polypoid hyperplastic and thickened regions of the cystic lining of Radicular Cysts (RCs) and Dentigerous Cysts (DCs) [5]. Moreover, mucous cell differentiation has been observed in the cystic lining of various Odontogenic Cysts (OCs) [6] and tumours [7], and in various salivary gland lesions.
In the present review, effort has been made to comprehensively discuss various aspects like histogenesis, theories and diagnostic importance of mucous prosoplasia in different oral pathologies.
Histopathogenesis of mucous cell prosoplasia
Mucous cell prosoplasia is seen in various OCs comprising RCs [5], residual cyst [8], DCs [5,6], odontogenic keratocyst [4,8] and in ameloblastomas [7]. Takeda Y et al., have examined 361 OCs and discovered that mucous cells were present in 18.1% of RCs, 23.8% of DCs and 26.9% of primordial cysts [5]. In a study by Cabber F et al., mucous cell prosoplasia was seen in 11.9% of dental follicle specimens [6]. Moreover, it is fascinating to note that the presence of prosoplastic change in OCs such as RCs and DCs is inconsistent to that of such change elsewhere in the body. Thus, it seems that some factors prevailing in the OCs supports this prosoplastic change [4].
The mucous cells within different types of the OCs are characterized by the following three different phenotypes:
Type-1: It includes cells producing only acidic mucin and that stains blue on staining with combined Alcian Blue-Periodic Acid Schiff (AB-PAS) stain.
Type-2: It includes cells producing only neutral mucin and that stains magenta.
Type-3: It includes mucous cells producing both acidic and neutral mucin and that stains purple on staining with combined AB-PAS technique [9].
However, the origin of these mucous cells in the epithelium of OCs and tumours has been a topic of debate till date. Various theories have been advocated concerning the occurrence of mucous cells in the cystic epithelium. Few of the theories are as follows:
1. Lining of the antral cavity: Shear M in 1960 has suggested that the manifestation of mucous secreting cells in the lining epithelium of OCs can be attributed to the transplantation of these cells from an adjoining epithelium that normally shows presence of mucous cells; for example, the epithelial lining of the nasal and antral cavity [10].
2. Pluripotential embryological cells: Shear M in 1960 has also proposed that the mucous cells might appear in the lining epithelium of OCs as a result of the embryological pluripotent cells in the residual cells from which the cysts are thought to arise [10]. Browne RM during a histologic study of the walls of 638 OCs observed a disparity in the incidence of mucous cells in various types of OCs [4]. This contradicts the pluripotential embryological cell hypothesis for origin of mucous cells in OCs. Presuming the origin of mucous cells in OCs from pluripotent embryological residual cells, their incidence in the cystic lining of various OCs should be equal.
3. Theory of transdifferentiation: Hodson JJ in 1956 proposed that the presence of mucous secreting cells in the cystic lining epithelium might be an outcome of prosoplastic modification of normal squamous cells of the lining epithelium into mucous secreting cells in response to change in their environment [11]. Biochemical analysis of the cystic contents of various cysts by Toller PA showed that the soluble protein contents were identical in RCs and DCs but different in odontogenic keratocyst [12]. Thus, the alteration in the incidence of mucous cells in these cysts may be due to such environmental inconsistencies. Also, the keratin layer lining the cystic epithelium of odontogenic keratocyst justifies the low prevalence of mucous prosoplasia as it may guard the epithelial cells from the inductive environmental provocations that may lead to cellular transdifferentiation [4].
Other than the above mentioned theories, some authors also found remarkable association between mucous and vacuolated cells. Shear N identified the presence of these vacuolated cells in relation with the mucous cells in the cystic lining of RC [10]. A similar result was found by Slabbert et al., in his research work in RC and residual cyst [13]. Fell H and Mellanby E portrayed a comparable kind of association between the mucous cells and vacuolated cells in the embryonic chicken skin developed in presence of excess vitamin A. Such changes depend on the level of differentiation of the epidermal cells. Cells that are deeply positioned in the epithelium display tonofibrils getting changed into irregular and darkly stained granules. Fell HA stated that in the initial progression of the mucous prosoplastic alteration, the keratinocytes become vacuolated and then the mucin granules amass within the vacuolated cells leading to the development of mucous cells [14,15]. Slabbert H et al., contributed the term ‘intermediate cells’ for these vacuolated cells [13]. Thus, these vacuolated cells signify a maiden step in the histogenesis of mucous prosoplastic modification [13].
Moreover, authors find a remarkable similarity between Glandular Odontogenic Cyst (GOC) and other OCs with mucous prosoplasia. However, it has been evident that the number and frequency of mucous cells is much higher in GOC than the other OCs with mucous prosoplasia suggesting that the mucous cells in GOC are common cystic lining components and not a result of transdifferentiation [7].
Diagnostic importance of mucous cell prosoplasia in oral lesions
Browne RM in one of his studies on the OCs noticed an increasing occurrence of mucous cells in RCs and DCs with increasing age of the patient indicating that the presence of mucous cell prosoplasia is associated with the age of the cystic lining [4].
It is a known fact that Mucoepidermoid Carcinoma (MEC) is the most common malignant salivary gland tumour [16]. One of the theories elucidating the origin of central MEC suggests that it can arise from the neoplastic transformation of the cystic lining of pre-existing OCs and tumours [17]. Kochaji N et al., demonstrated mucous prosoplasia in the epithelial component of DC, in a reported case of central MEC of maxilla [18]. Moreover, the presence of MAML2 rearrangement in the epithelial lining of OCs with mucous prosoplasia and central MEC suggests that central MEC might progress from the OCs with mucous prosoplasia [16].
Another entity is Warthin’s tumour, which consists of two components: an epithelial and a stromal component. The epithelium component of the tumour is shown to be a frequent site of various types of transdifferentiation [19]. Eveson JW and Cawson RA et al., studied around 278 cases of Warthin’s tumour and reported mucous cell prosoplasia in around 15% of the cases [20]. Seifert G et al., studied around 275 Warthin’s tumour of parotid gland and classified these tumours into four subtypes [21]. Type 4 included tumours with epithleial components showing characteristic squamous metaplasia and mucous prosoplasia [19]. Metamorphosis of Warthin’s tumour into malignancy is a very rare but well-documented phenomenon [22]. Ruebner B and Bramhall JL were the first to present a case of malignant transformation of Warthin’s tumour [23]. Later, various such transformations within the Warthin’s tumour have been reported including squamous cell carcinoma, adenocarcinoma, undifferentiated carcinoma and mucoepidermoid carcinoma [24]. Explaining the pathogenesis of this conversion into malignancy, Seifert G observed a persistent transformation of a typical tumour cell in the epithelial component of Warthin’s tumour to a squamous cell, mucous cell and a neoplastic cell [25]. Thus, such type of cellular transdifferentiation within the Warthin’s tumour should be well acknowledged for contradistinction from various other malignancies.
Oncocytoma is a tumour that mainly consists of oncocytes that are basically epithelial cells with abundant mitochondria, giving a granular cytoplasmic appearance [26]. Xiaoyan L et al., in his study detected few mucocytes in oncocytic carcinoma [27]. This suggests that the presence of mucous cells in the oncocytic neoplasms increases the speculation of oncocytic MEC, a rare morphological form of MEC [26].
Differentiation of GOC from other odontogenic cysts with mucous prosoplasia is quite challenging for a pathologist, as the presence of mucous producing cells and duct like structures is not an infrequent feature of GOC. It is, thus, of immense importance to differentiate between the two since the treatment and prognostic inference varies [9,28].
Identification of Mucous Prosoplasia
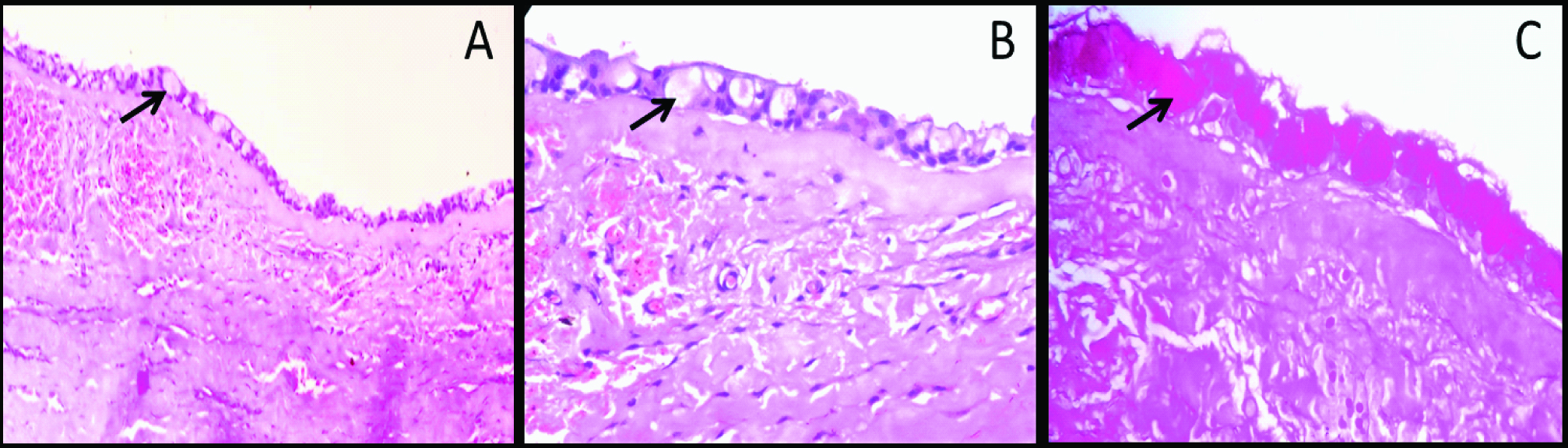
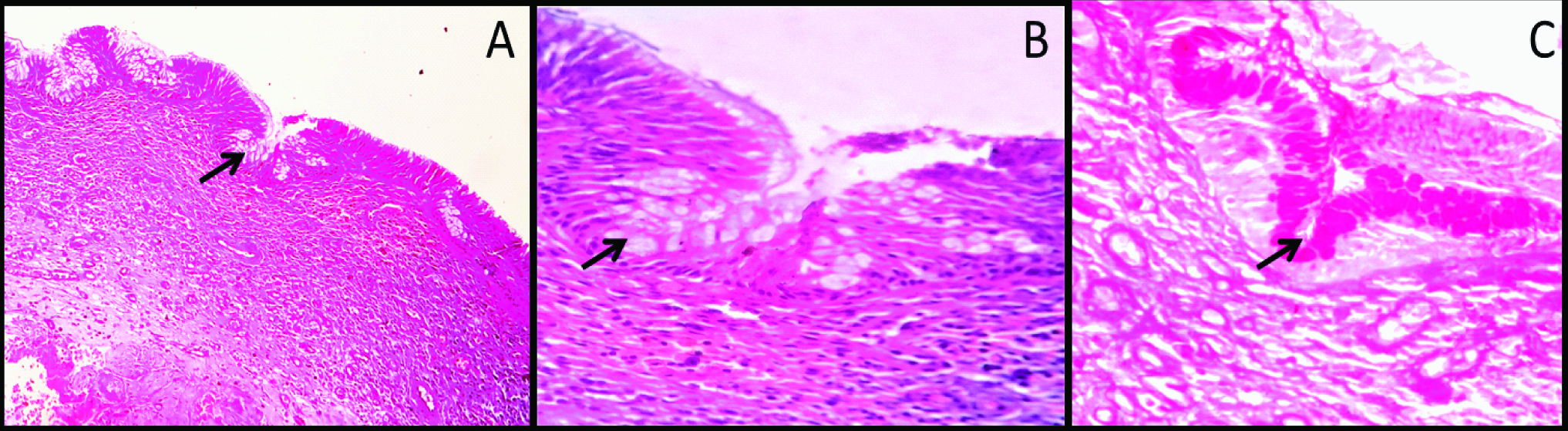
Identification of mucous prosoplasia on histopathology sections is not difficult. This phenomenon usually manifest histopathologically as polygonal to cuboidal cells with basophilic to amphophilic cytoplasm suggesting mucin content in the cell. They are usually present in the groups or isolated in the epithelial linings. Occasionally these cells are columnar and exhibit cilia on the outer surface [Table/Fig-1,2 and 3]. However, population of mucous cells in the lining epithelium sometimes may be very small and cannot be viewed easily. In such situations, special stains like alcian blue, per iodic acid Schiff, Mayer mucicarmine, etc., can be used [Table/Fig-1,2 and 3] [29]. In addition to this, immunohistochemical studies by Guler N et al., have also shown strong expression of proliferative markers like Ki-67 and MCM-2 in mucous cell prosoplasia [8].
Photomicrograph showing dentigerous cyst with characteristic epithelial lining: (a) The epithelial lining is showing two to three cell layer thickness (100X); (b) High power magnification showing round to polygonal mucous cells in the lining epithelium (400X); (c) Mucous cells showing intense staining with mucicarmine stain (400X).
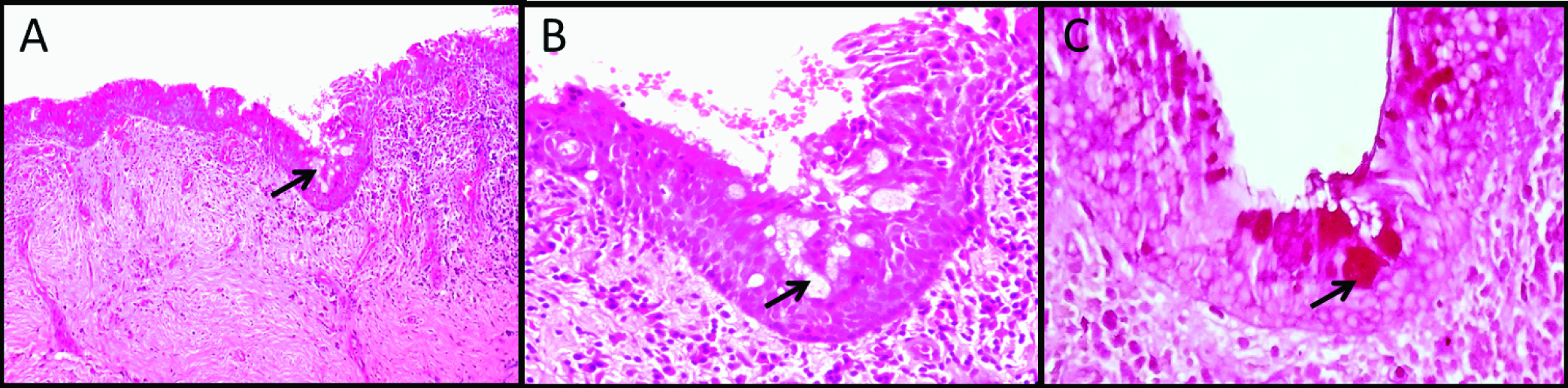
Photomicrograph showing odontogenic keratocyst with characteristic epithelial lining: (a) The epithelial lining is showing parakeratinized epithelium with uniform thickness (100X); (b) High power magnification showing round to polygonal cells with basophilic cytoplasm (400X); (c) Focal areas of intense mucicarmine stain (400X).
Photomicrograph showing radicular cyst with characteristic epithelial lining: (a) The cyst is characterized by inflammatory cell infiltration in the connective tissue wall (100X); (b) High power magnification showing group of round to polygonal mucous cells in the lining epithelium (400X); (c) Mucous cells showing intense staining with mucicarmine stain (400X).
Conclusion
In conclusion, mucous prosoplasia is, although rare but important event in various oral pathologies, especially odontogenic cysts. Various hypotheses have been proposed for its histogenesis but still exact mechanism is not clear. As some of the lesions are prognostically important, oral pathologies with mucous prosoplasia require careful diagnosis because of overlapping histopathological appearances.
[1]. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, Molecular Biology of the Cell 2002 4th edNew YorkGarland Science [Google Scholar]
[2]. Slack JMW, Metaplasia and transdifferentiation: from pure biology to the clinicNature Reviews Molecular Cell Biology 2007 8(5):369-78. [Google Scholar]
[3]. Bignold Leon P, Brian LD Coghlan, Hubertus PA Jersmann, David Paul von Hansemann: contributions to oncology 2007 Springer [Google Scholar]
[4]. Browne RM, Metaplasia and degeneration in odontogenic cysts in manJ Oral Path 1972 1:145-58. [Google Scholar]
[5]. Takeda Y, Oikawa Y, Furuya I, Satoh M, Yamamoto H, Mucous and ciliated cell metaplasia in epithelial lining of odontogenic inflammatory and developmental cystsJ Oral Sci 2005 47:77-81. [Google Scholar]
[6]. Cabber F, Guler N, Comunoqlu N, Sencift K, Coloqlu S, Determination of potential cellular proliferation in the odontogenic epithelia of the dental follicle of the asymptomatic impacted third molarJ Oral Maxillofac Surg 2008 66:2004-11. [Google Scholar]
[7]. Wilson D, Walker M, Aurora N, Moore S, Ameloblastoma with mucous cell differentiationOral Surg Oral Med Oral Pathol Oral Radiol Endod 2001 91:576-78. [Google Scholar]
[8]. Guler N, Comunoglu N, Cabbar F, Ki-67 and MCM-2 in dental follicle and odontogenic cysts: The effects of inflammation on proliferative markersScientific World Journal 2012 18:946060 [Google Scholar]
[9]. Carim R, Mahomed F, A comparative histochemical study of mucous cells in odontogenic cystsISRN pathology 29 2013 [Google Scholar]
[10]. Shear M, Secretory epithelium in the lining of dental cystsJournal of the Dental Association of South Africa 1960 15:117-22. [Google Scholar]
[11]. Hodson JJ, Mucoepidermoid odontogenic cysts of the jaws with special reference to those in the mandibleProceedings to the Royal society 1956 B49:637-39. [Google Scholar]
[12]. Toller PA, The osmolality of fluids from cysts of the jawsBritish Dent J 1970 129:275-78. [Google Scholar]
[13]. Slabbert H, Shear M, Altini M, Vacuolated cells and mucous metaplasia in the epithelial linings of radicular and residual cystsJ Oral Pathol Med 1995 24:309-12. [Google Scholar]
[14]. Fell HB, Mellanby E, Metaplasia produced in cultures of chick ectoderm by high vitamin AJournal Physiology 1953 119:470-88. [Google Scholar]
[15]. Fell HB, The effect of excess vitamin A on cultures of embryonic chicken skin explanted at different stages of differentiationProc royal society 1957 146:242-56. [Google Scholar]
[16]. Prokopios PA, Rebecca NW, Joaquin JG, Joannis GK, Fluorescence in-situ hybridization identifies mastermind-like 2 (MAML2) rearrangement in odontogenic cysts with mucous prosoplasia: a pilot studyHistopathology 2015 66:791-97. [Google Scholar]
[17]. Dain CP, Thomas JP, Ambika K, Heera R, Rency K, Manoj JM, Central mucoepidermoid carcinoma of the mandible- From a histopathologic perspectiveJournal of Oral and Maxillofacial Surgery, Medicine and Pathology 2015 27:147-50. [Google Scholar]
[18]. Kochaji N, Gooossens A, Bottenberg P, Central mucoepidermoid carcinoma: case report, literature review for missing and available information and guideline proposal for coming case reportsOral Oncology extra 2004 40:95-105. [Google Scholar]
[19]. Weiss LM, Brodsky GL, Adenolymphoma with massive necrosis and squamous metaplasiaActa Pathol Jpn 1984 34:1469-74. [Google Scholar]
[20]. Eveson JW, Cawson RA, Warthin’s Tumour (cystadenolymphoma) of salivary gland- a clinicopathological investigation of 278 casesOral Surg Oral Med Oral Pathol 1986 61:256-69. [Google Scholar]
[21]. Seifert G, Bull HG, Donath K, Histologic subclassification of the cyst adenolymphoma of the parotid gland. Analysis of 275 casesVirchows Arch A Pathol Anat Histol 1980 388:13-38. [Google Scholar]
[22]. Ellis GL, Auclair PL, Tumours of the Salivary Glands 1996 Washington DCArmed Forces Institute of Pathology [Google Scholar]
[23]. Ruebner B, Bramhall JL, Malignant papillary cystadenomalymphomatosumArch Pathol 1960 69:110-17. [Google Scholar]
[24]. Nagao T, Sugano I, Ishida Y, Tajima Y, Furuya N, Kondo Y, Mucoepidermoid carcinoma arising in Warthin’s tumour of the parotid gland: report of two cases with histopathological, ultrastructural and immunohistochemical studiesHistopathology 1998 33:379-86. [Google Scholar]
[25]. Seifert G, Bilateral mucoepidermoid carcinoma arising in bilateral pre-exisitng Warthin tumour of parotid glandOral oncology 1997 33:284-87. [Google Scholar]
[26]. Victoria C, Arash R, David S, Meera M, Cutaneous oncocytoma- a report of three cases and review of the literatureJournal of cutaneous pathology 2007 34:355-59. [Google Scholar]
[27]. Xiaoyan L, Parviz H, Charles SC, Xiangdong X, A rare case of exclusively oncocyticmucoepidermoid carcinoma with MAML2 translocationRare tumours 2016 8:66-68. [Google Scholar]
[28]. Hussain K, Edmondson HD, Browne RM, Glandular odontogenic cysts. Diagnosis and treatmentOral Surg Oral Med Oral Pathol Oral Radiol Endod 1995 79:593-602. [Google Scholar]
[29]. Wick MR, Histochemistry as a tool in morphological analysis: a historical reviewAnnals of diagnostic pathology 2012 16:71-78. [Google Scholar]