This case report presents a case of drug induced gingival overgrowth in a 28-year-old female patient with history of Coronary Artery Disease (CAD) and was prescribed digoxin in combination with furosemide and acitrom for the same. On clinical examination, the patient presented with severe gingival overgrowth. The volume of enlargement seen did not correlate solely with the diagnosis of inflammatory Gingival Enlargement (GE), hence an added drug induced component to the Gingival Overgrowth (GO) was suspected. It was decided to treat the condition using initial therapy {meticulous Scaling and Root Planning (SRP)} followed by a period of observation and maintenance. On recall, since there was no marked improvement, surgical intervention was planned. Periodic treatment combining surgery and maintenance ultimately produced the desired results clinically. Thus, this article within limits highlights that because of time-relationship between the starting of the medication that is digoxin and manifestation of GO, a causal relationship is likely.
Case Report
A 28-year-old female reported with the chief complaint of swollen and bleeding gums since two years. Initially, the patient noticed mild swelling in the gingiva which gradually increased in size and became more pronounced over the last three to four months. The patient also complained of displacement of teeth and generalised increased spacing between teeth.
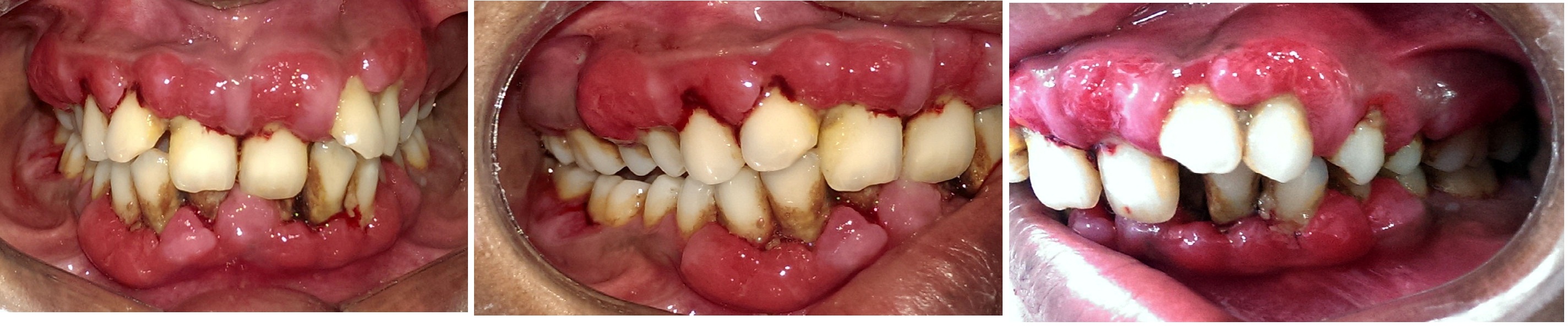
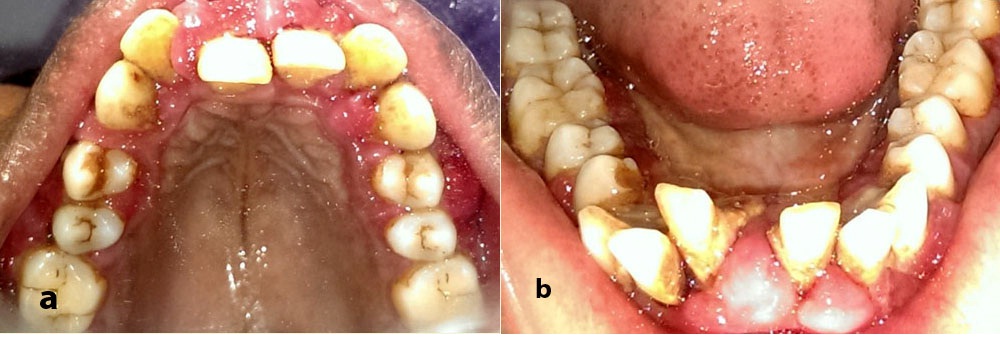
Evaluation of medical history revealed that she was a known case of CAD and had undergone cardiac surgery two years ago. Drugs prescribed postsurgery were tab digoxin 0.25 mg once daily, tab frusemide 40 mg once a week, tab acitrom 2 mg once daily. Patient had noticed mild swelling in her gingiva a couple of months after she started medications postcardiac surgery [Table/Fig-1a-c]. Due to the swelling and excessive bleeding while brushing, patient’s oral hygiene maintenance was hampered causing her to refrain from tooth brushing. Associated pathologic migration of anterior teeth was also noticed [Table/Fig-2a,b]. Patient had developed mouth breathing habit because of excessive GO and displaced teeth.
Periodontal examination revealed generalised reddish gingiva with diffuse type of GO covering one third of the tooth structure in anterior region which was more pronounced on the labial aspect of the teeth. The gingiva was soft and edematous in consistency, suggesting an added inflammatory component. Generalised combined probing pocket depths ranging from 5 mm-11 mm was noted. All teeth were mobile with advanced mobility (Miller’s Class III mobility) in 25, 31, 32, 41, 42 and all molars presented with furcation involvement ranging in severity from Glickman’s Grade II to Grade III.
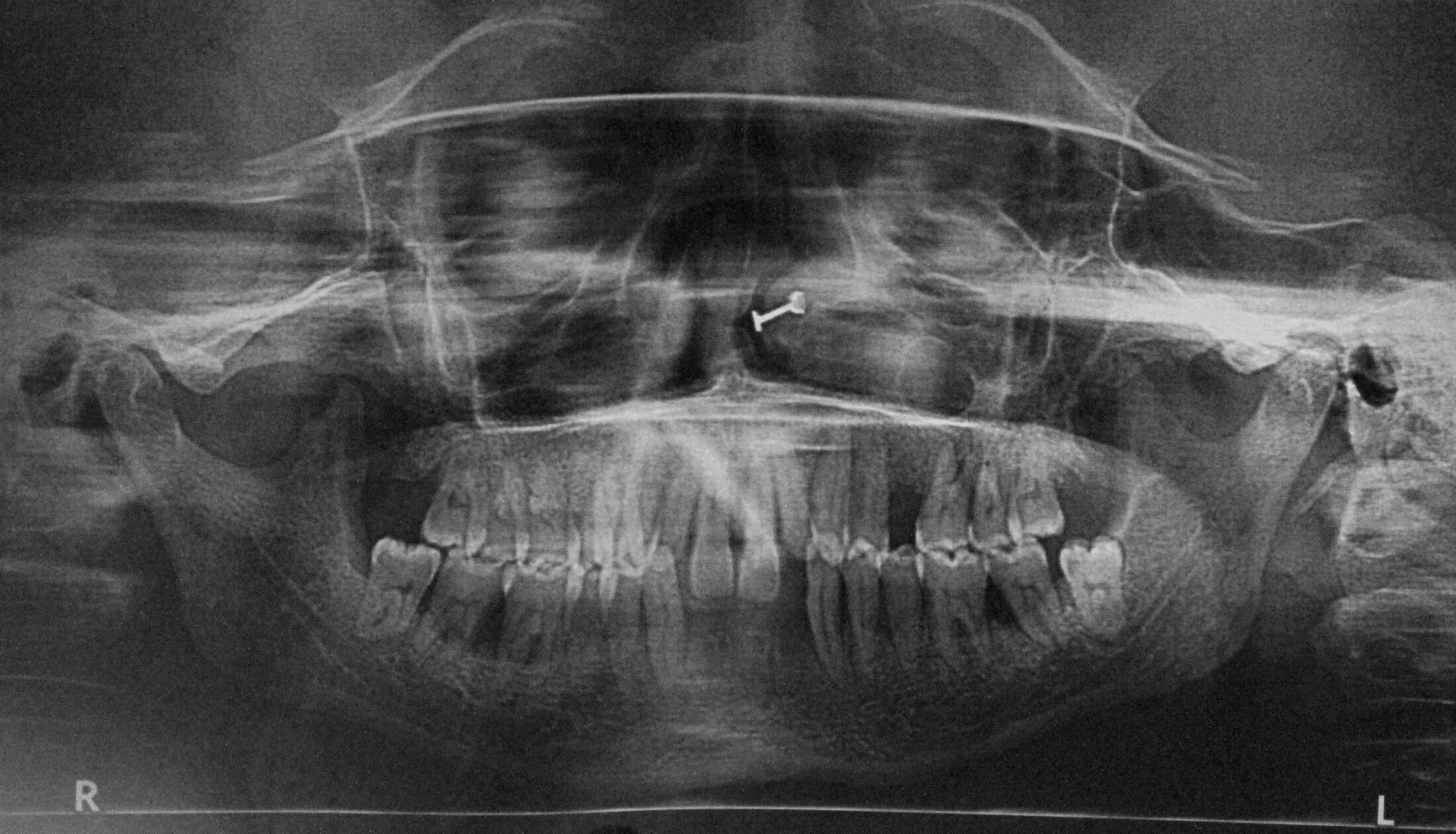
Orthopantomograph [Table/Fig-3] and full mouth intraoral periapical radiographs of the patient revealed moderate to severe bone loss around all teeth.
Clinically, the case was provisionally diagnosed as generalised Aggressive Periodontitis (AgP) associated with generalised Drug Induced Gingival Overgrowth (DIGO) and the differential diagnosis was generalised severe chronic periodontitis associated with generalised chronic inflammatory gingival overgrowth.
Case Management
With medical consent, tab. acitrom was discontinued five days prior to every surgical procedure and resumed three days after so as to avoid surgical complications.
Preliminary phase treatment included extraction of 25, 31, 32, 41 and 42 as their periodontal prognosis was hopeless.
The initial periodontal therapy comprising of SRP was performed quadrant wise and combination antimicrobial therapy (ciprofloxacin and tinidazole, 500 mg b.i.d for seven days) was prescribed. This was followed by one month period of observation and maintenance. During the one month follow up period, patient was recalled every week for repeat SRP and re-evaluation. However, GO persisted despite repeated SRP, hence surgical intervention was planned.
At first surgical visit, external bevel gingivectomy was performed in relation to maxillary anterior and premolar region to reduce bulk of the overgrown tissue in order to facilitate oral hygiene maintenance. Surgical site was cauterized using electrocautery to control bleeding and meticulous SRP was performed. Excised tissue was sent for histopathological examination.
Following healing after the gingivectomy procedure, sextant wise full mouth periodontal flap surgeries were performed using internal bevel incision in order to treat the persistent deep periodontal pockets.
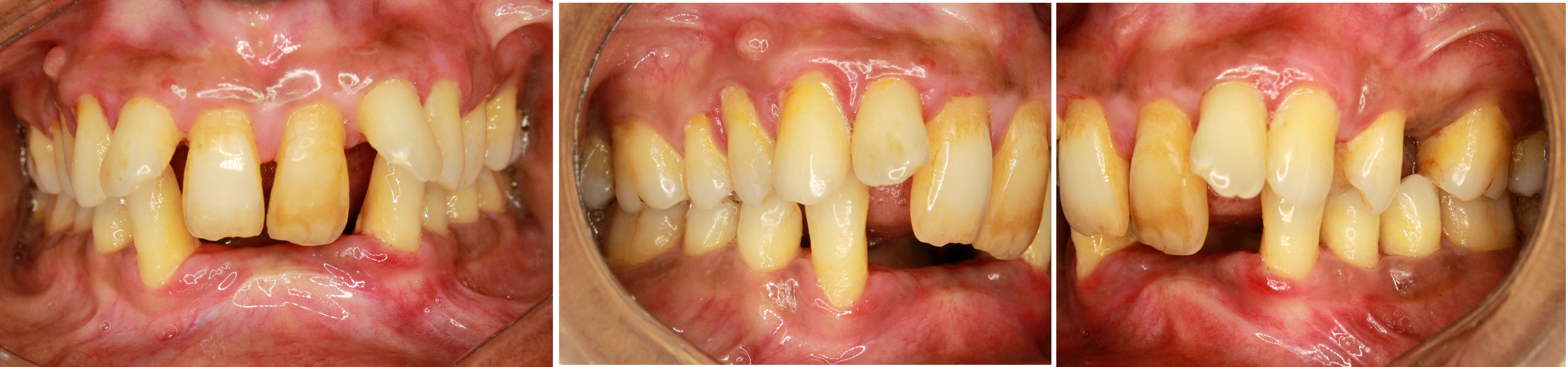
On examination at three month review, probing pocket depth was generally reduced to 3 mm. However, generalised mild recession was noted. Patient was followed up at regular intervals and oral hygiene was reinforced at every visit. After completion of six months of surgery, satisfactory periodontal condition was confirmed [Table/Fig-4a-c]. Patient was followed up for a period of one year and no recurrence was noted.
Histopathologic Examination
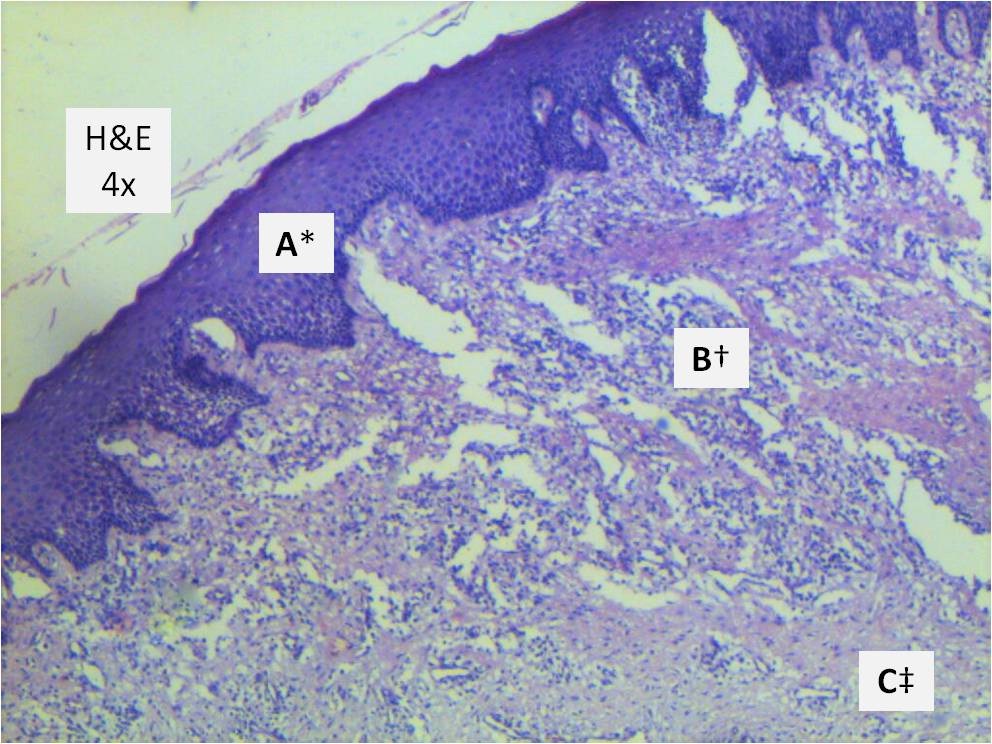
Histopathology examination [Table/Fig-5] of Haematoxylin and Eosin (H&E) stained section revealed hyperplastic parakeratinised stratified squamous epithelium. Underlying stroma showed sparse fibrous connective tissue with plenty of chronic inflammatory cell infiltrate; predominantly plasma cells and lymphocytes and few macrophages. The histopathologic diagnosis was chronic gingival hyperplasia.
a) Preoperative photo showing severe gingival overgrowth-anterior view. b) Preoperative photo-right lateral. c) Preoperative photo-left lateral.
Preoperative photo-occlusal view.
a) Six months after surgical therapy-anterior view. b) Six months after surgical therapy-right lateral. c) Six months after surgical therapy-left lateral.
Photomicrograph of H&E stained histological section under 4X magnification.
* Parakeratinized stratified squamous epithelium
† Superficial connective tissue showing dense inflammatory cell infiltrate
‡ Cellular connective tissue
Discussion
GO or GE can result due to various etiological factors one being an undesirable side effect of various drugs. DIGO is considered under type B adverse drug reactions as the effect is not predictable from known pharmacological properties of the implicated drugs [1]. Literature most commonly links drugs like anticonvulsants, calcium channel blockers and immunosuppressants to GO [2,3]. The presence of GE causes difficultly in routine plaque control, leading to new bacterial niches often resulting in secondary inflammation and enlargement. Thus the dual etiology in these cases should be understood and treated adequately.
In the present case, the patient reported with GO associated with severe periodontitis and had no other underlying systemic condition except presence of CAD and was on medication for the same. A literature survey was conducted to find if there was any data associating GO to any of the two drugs prescribed to the patient post cardiac surgery. This brought into focus that there indeed was scanty data linking the drug digoxin to GO in isolated instances [4,5].
Digoxin is frequently prescribed in patients with atrial fibrillation/ flutter. Digoxin has rarely been reported to cause of GO [4]. Nevertheless a prevalence of 0.02% of gingival hyperplasia has been reported with digoxin use [4]. The Food & Drug Administration (FDA) reported 36,894 digoxin drug adverse reactions between January 2004 and October 2012, of which six individuals taking digoxin reported GO [5]. Often FDA only receives reports of the most critical and severe cases; thus, these figures may under represent complication rate of the medication. Percentage of digoxin patients where gingival hyperplasia was a reported side effect was 0.0163% [5].
Cardiac glycosides like digoxin besides specific inhibition of sodium pump can stimulate cell growth by Mitogen-Activated Protein Kinase (MAPK) pathway activation in various cell types and can effect cytosolic signalling in hormone like manner [6]. MAPK activation especially activation of extracellular signal regulated protein kinase (ERK1/2) is critical for cellular proliferation and differentiation [7]. A recent study showed that low concentrations of digoxin can activate proliferation of human fibroblasts via Na+, K+-ATPase complex as a transducing receptor [8]. Thus, it may be suggested that MAPK pathway activation via Na+, K+-ATPase complex receptor could be probable molecular mechanism for such an overgrowth.
Certain common diagnostic clinical features of DIGO’s have been previously outlined [9]. GE’s clinically manifest within one to three months after initiation of treatment with associated medication [10]. In the present case also, the patient had noticed gingival swelling a couple of months after beginning medications. Another finding consistent with our case was that the overgrowth was first noticed in anterior region and was also more pronounced in same region. The relationship between plaque, gingival inflammation and DIGO has been confirmed [11]. Since, patient had undergone routine dental therapy including SRP as a part of clearance for cardiac surgery, the volume of enlargement seen does not seem to correlate solely with diagnosis of inflammatory GE. Hence, we suspected an added drug induced component to the GO.
The GO was also associated with AgP. AgP clinically presents as severe, rapidly progressive form of periodontitis and is characterized by an early age of clinical manifestation [12]. Few case reports have shown association of GE with AgP [13,14]. Here, the AgP may not be a separate disease entity but the periodontitis may have progressed more rapidly because presence of local factors confounded by mouth breathing and compromised oral hygiene maintenance.
Most effective treatment for DIGO is withdrawal or substitution of medication [3,15]. However, physicians may sometimes be reluctant to substitute an effective drug regimen as the gravity of the underlying systemic conditions may far outweigh the need for drug substitution for localized intraoral side effects. In such cases, we have to rely on other modes of case management like surgical intervention and effective plaque control. Although, many patients respond favourably to nonsurgical treatment and appropriate drug substitution, a significant number of them require surgical removal of overgrown tissues. In present case, since drug substitution was not an option considering the underlying systemic condition; the case was managed by surgical intervention. Since, the prescribed drug may be a suggested causative agent for GO, recurrence can be anticipated.
Conclusion
Thus, it would be befitting within limits to conclude that though there is a paucity of data linking the drug digoxin to DIGO, it would surely add to our repertoire of clinical knowledge if this can indeed occur and to what extent the drug can affect the same. Although, activation of MAPK pathway has been suggested more research at molecular level is required to clearly establish the underlying pathogenesis involved in digoxin induced GO; thus paving the way for establishment of pertinent information for designing future preventive and therapeutic strategies.
[1]. RA Seymour, PA Heasman, IDM Macgregor, Adeverse drug reactions and the periodontal tissues. In: drugs, diseases, and the periodontium 1992 New YorkOxford University Press:77 [Google Scholar]
[2]. O Kimball, The treatment of epilepsy with sodium dipenylhydantoinateJAMA 1939 112:1244-45. [Google Scholar]
[3]. A Dongari-Bagtzoglou, Drug-associated gingival enlargementJ Periodontol 2004 75:1424-31. [Google Scholar]
[4]. eHealthMe Personalized Health Information [Internet]. Review: Could Digoxin cause Gingival hyperplasia?; c2017 [updated 2017 Feb 01; cited 2017 Feb 01]. Available from: http://www.ehealthme.com/ds/digoxin/gingival%20hyperplasia/ [Google Scholar]
[5]. FDA Research Report [Internet]. Is gingival hyperplasia a side effect of Digoxin?; Factmed, Inc; c2014 [updated 2016 Aug 03; cited 2017 Jan 30]. Available from: http://factmed.com/study-DIGOXIN-causing-GINGIVAL%20HYPERPLASIA.php [Google Scholar]
[6]. Z Xie, A Askari, Na+, K+-ATPase as a signal transducerEur J Biochem 2002 269(10):2434-39. [Google Scholar]
[7]. L Chang, M Karin, Mammalian MAP kinase signaling cascadesNature 2001 410(6824):37-40. [Google Scholar]
[8]. K Winnickaa, K Bielawskib, A Bielawskab, W Miltykb, Dual effects of ouabain, digoxin and proscillaridin A on the regulation of apoptosis in human fibroblastsNat Prod Res 2010 24(3):274-85. [Google Scholar]
[9]. A Mariotti, Dental plaque-induced gingival diseasesAnn Periodontol 1999 4:07-19. [Google Scholar]
[10]. SJ Meraw, PJ Sheridan, Medically induced gingival hyperplasiaMayo Clin Proc 1998 73:1196-99. [Google Scholar]
[11]. JS Ellis, RA Seymour, JG Steele, P Robertson, TJ Butler, JM Thomason, Prevalence of gingival overgrowth induced by calcium channel blockers: a community-based studyJ Periodontol 1999 70:63-67. [Google Scholar]
[12]. GC Armitage, Development of a classification system for periodontal diseases and conditionsAnn Periodontol 1999 4:01-06. [Google Scholar]
[13]. P Casavecchia, MI Uzel, A Kantarci, H Hasturk, S Dibart, TC Hart, Hereditary gingival fibromatosis associated with generalized aggressive periodontitis: A case reportJ Periodontol 2004 75:770-78. [Google Scholar]
[14]. R Chaturvedi, Idiopathic gingival fibromatosis associated with generalized aggressive periodontitis: a case reportJ Can Dent Assoc 2009 75:291-95. [Google Scholar]
[15]. PM Camargo, PR Melnick, FQ Pirih, R Lagos, HH Takei, Treatment of druginduced gingival enlargement: aesthetic and functional considerationsPeriodontol 2001 27:131-38. [Google Scholar]