Colorectal cancer is the third most common cancer in men and second most common cancer in women, worldwide [1]. Increased risk of colon cancer has been linked with ingestion of red meat, processed meat and low vegetable and fibre consumption. Red meat and dairy products are rich in branched chain fatty acids which require an enzyme AMACR/P504S for their oxidation. It is an enzyme bimodally located in mitochondria and peroxisomes; required in β-oxidation of branched chain fatty acids [2]. Increased AMACR expression is seen in many premalignant conditions and various carcinomas such as prostatic adenocarcinoma, colorectal carcinoma, hepatocellular carcinoma and renal cell carcinoma [3-6]. Increased expression of AMACR in colorectal premalignant lesions and carcinomas suggests that it has a possible role in colorectal carcinogenesis [7].
It is possible that AMACR induced by dietary factors plays a role in regulating cell proliferation as overexpression of acyl-CoA oxidase, regulated by AMACR, can transform cells in vitro. Also, it has been found that overexpression of AMACR by transfection of plasmid cDNA expressing the full-length gene results in increased overall proliferation rate of prostate tumour cells [7]. Another hypothesis says that overexpression of AMACR in the development of cancer may play a role in providing energy for the neoplastic cells [8].
The aim of the study was, to study the expression of AMACR in colorectal neoplasia and its correlation to histological grade and stage of the colorectal malignancy. The study is clinically relevant as AMACR may be used as a prognostic marker and may have a possible role in targeted therapy of the disease.
Materials and Methods
This prospective study was conducted in the Department of Pathology, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha, India, from September 2013 to August 2015. The research was done after obtaining clearance from the Ethical Committee of the Institution. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975 that was revised in 2000.
A total of 56 cases were analysed in the study, which included 44 cases of adenocarcinoma and 12 cases of adenoma. The age of patients ranged from 21-83 years with a male to female ratio of 2:1. A tissue microarray was prepared by manual method.
Preparation of tissue microarray: Tissue blocks were taken and fresh full face 3 μm HandE sections were cut and reviewed under the light microscope. The area of interest was identified and marked on the glass slide. Corresponding area on the tissue block was matched and marked. A bone marrow trephine biopsy needle was used to punch out tissue cores from the donor blocks. For this, the stylet was removed from the cannula handle and the cannula was inserted within the marked area on the tissue block upto 5 mm depth. Then the cannula was taken out and the stylet was inserted inside the cannula to gently push out the tissue core. On a clean table, an area was marked with 30 dots using a pencil as per the plan of tissue microarray layout. An adhesive transparent tape was applied on the area so that the dots were still visible. Now, one by one the tissue cores were picked by forceps and placed over the dots according to the order of numbering of blocks. The tissue cores got glued to the adhesive tape. Once all the tissue cores were placed an L-mould was kept over the marked area. Molten wax was gradually poured inside the mould. After the wax solidified, the mould was removed and the block was kept on the cold plate. When the block was ready thin; 3 μ sections were cut and taken on albumin coated slides.
AMACR expression was studied by standard (IHC) methods. IHC evaluation of AMACR was done on formalin fixed paraffin embedded tissue sections (3 μm-4 μm thick) on poly-L lysine coated slides by using polymer two step indirect methods using antibodies and chemicals obtained from Biogenex, USA. (clone-AN538-5M).
Assessment of staining: The assessment of IHC staining was carried out by three independent pathologists. A semi quantitative system based on intensity of reaction product and percentage of cells showing cytoplasmic positivity were used.
Staining intensity was scored as negative (0), weak (1+), moderate (2+), and strong (3+).
The extent of staining was represented as percentages of positive staining areas in relation to the whole carcinoma area. It was scored as 0-5% (Score 0), 6-20% (Score 1), 21-40% (Score 2), 41-60% (Score 3), 61–80% (Score 4), and 81-100% (Score 5) [Table/Fig-1a-d].
Photomicrograph showing different scores of expression of AMACR in colorectal neoplasm, negative a); mild b); moderate c); and strong d).[20X].
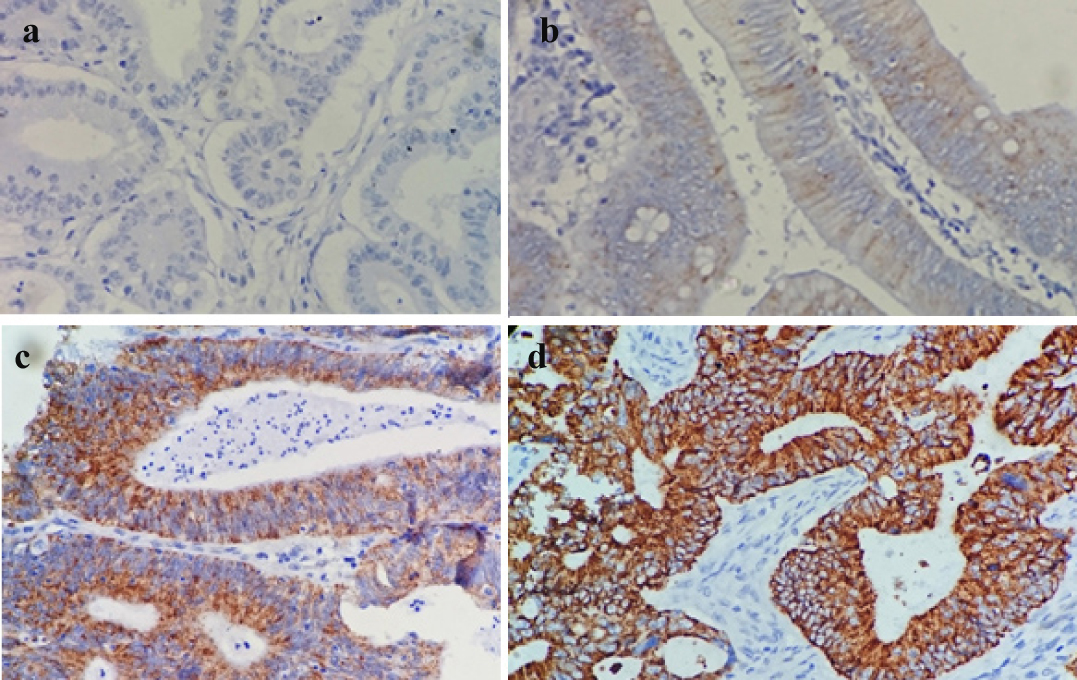
The product of intensity and extent of staining scores were used as final staining scores (0-15). For the purpose of statistical analysis, tumours having a final staining score of ≥1 were considered to be positive. The final scores of AMACR expression were graded as negative (Score 0), poor (Score 1-5), moderate (Score 6-10), strong (Score 11-15).
Statistical Analysis
Statistical analysis was done to find out the significance of AMACR expression in adenoma and carcinoma and association between AMACR expression and various histopathological parameters. The relationship between qualitative parameters was determined using the Chi-square test. Statistical significance was defined as p<0.05.
Results
A total of 56 cases were taken including colorectal adenomas, adenocarcinoma and one normal colonic tissue used as control tissue. The age range of patients was 21-83 years with a mean age of 50.51 years (SD=11.12). Out of all cases 21 (47.7 %), 21 (47.7%) and 2 (4.5%) were Grade 1, 2 and 3 respectively. A 15.9% (7/44), 40.9% (18/44) and 43.18% (19/44) cases were in Stage I, II and III respectively. Maximum numbers of cases were Stage IIA and only two cases were of Stage IIB. For the purpose of analysis Stage I and II tumours were combined together.
A 56.81% (25/44) cases were node negative (N0) and 43.18% (19/44) cases were node positive (N1 and N2). While doing statistical analysis N1 and N2 were grouped together as node positive cases.
There were 12 (20%) cases of adenoma comprising of 41.66% (5/12) villous adenoma, 33.33% (4/12) tubular adenoma and 25% (3/12) tubulovillous adenoma.
AMACR expression in normal colonic tissue, adenoma and carcinoma: There was no AMACR expression in normal colonic tissue. Out of 12 cases of adenoma 25% (3/12) were negative; 50% (6/12) showed poor expression, 25% (3/12) had moderate expression and none showed strong expression. A total of 34.1% (15/44) cases of carcinoma were negative; 45.45% (20/44) had poor; 13.63% (6/44) had moderate and 6.81% (3/44) had a strong expression. Statistical analysis revealed that there is no significant difference of expression between adenoma and carcinoma cases (p>0.05).
AMACR expression in carcinoma of different grades is shown in [Table/Fig-2]. Statistical analysis revealed that there was no significant difference of AMACR expression in different grades of the tumour (p>0.05) [Table/Fig-3].
AMACR expression in carcinoma of different grades.
| Grade | Negative | Poor | Moderate | Strong | Total |
|---|
| No. | % | No. | % | No. | % | No. | % | No. | % |
|---|
| G1 | 8 | 38.1 | 7 | 33.33 | 3 | 14.28 | 3 | 14.28 | 21 | 47.72 |
| G2 | 6 | 28.57 | 12 | 57.14 | 3 | 14.28 | 0 | 0 | 21 | 47.72 |
| G3 | 1 | 50 | 1 | 50 | 0 | 0 | 0 | 0 | 2 | 4.545 |
| TOTAL | 15 | 34.1 | 20 | 45.45 | 6 | 13.63 | 3 | 6.81 | 44 | 100 |
Association between AMACR expression and grade of carcinoma.
| Grade | Positive | Negative | Total | Chi-square and p-value |
|---|
| No. | % | No. | % | No. | % |
|---|
| G1 | 13 | 62 | 8 | 38 | 21 | 50 | χ2 = 0.4285p >0.05 |
| G2 | 15 | 71.4 | 6 | 28.6 | 21 | 50 |
| TOTAL | 28 | 66.66 | 14 | 33.33 | 42 | 100 |
AMACR expression in colorectal carcinoma in different AJCC stages: Strong AMACR expression was noted in significant number of cases in Stage I and II, whereas poor expression was seen in tumours of higher stages [Table/Fig-4].
AMACR expression in colorectal carcinomas in different AJCC stages.
| Stage | Negative | Poor | Moderate | Strong | Total |
|---|
| No. | % | No. | % | No. | % | No. | % | No. | % |
|---|
| I | 3 | 42.85 | 3 | 42.85 | 0 | 0 | 1 | 14.28 | 7 | 15.9 |
| IIA | 4 | 26.66 | 6 | 40 | 4 | 26.7 | 1 | 6.66 | 15 | 34.1 |
| IIB | 1 | 33.33 | 0 | 0 | 1 | 33.3 | 1 | 33.33 | 3 | 6.81 |
| IIIA | 1 | 50 | 1 | 50 | 0 | 0 | 0 | 0 | 2 | 4.54 |
| IIIB | 2 | 33.33 | 4 | 66.66 | 0 | 0 | 0 | 0 | 6 | 13.6 |
| IIIC | 4 | 36.36 | 6 | 54.54 | 1 | 9.09 | 0 | 0 | 11 | 25 |
| TOTAL | 15 | 34.09 | 20 | 45.45 | 6 | 13.6 | 3 | 6.81 | 44 | 100 |
Stage I and II (without nodal involvement) were considered together while performing the statistical analysis. Stage I and II showed 68% (17/25) AMACR expression. Stage III showed 63.15% (12/19) AMACR expression. We did not find any significant association between AMACR expression and stage (Chi-square=0.1125, p>0.05).
AMACR expression and nodal status: AMACR expression varied according to the nodal metastasis status or the tumours [Table/Fig-5]. There were 68% (17/25) node negative cases showing positive AMACR expression whereas, 63.15% (12/19) node positive cases showed AMACR expression. χ2 analysis showed that there is no significant association between AMACR expression and nodal status (Chi-square = 0.1125, p>0.05).
AMACR expression in different nodal stages of colorectal carcinomas.
| Stage | Negative | Poor | Moderate | Strong | Total |
|---|
| No. | % | No. | % | No. | % | No. | % | No. | % |
|---|
| N0 | 8 | 32 | 9 | 36 | 5 | 20 | 3 | 12 | 25 | 56.8 |
| N1 | 3 | 37.5 | 5 | 62.5 | 0 | 0 | 0 | 0 | 8 | 18.2 |
| N2 | 4 | 36.36 | 5 | 45.45 | 2 | 18.2 | 0 | 0 | 11 | 25 |
| TOTAL | 15 | 34.1 | 19 | 43.18 | 7 | 15.9 | 3 | 6.81 | 44 | 100 |
Discussion
AMACR has been found to be up regulated in various carcinomas and their precursor lesions, suggesting a role for this enzyme in the basic mechanism of the early stages of cancer formation [7].
AMACR expression in colorectal adenomas
The present study revealed increased AMACR expression in adenoma cases; with 75% (9/12) cases of adenoma showing positive expression. In the positive cases, there was weak expression in 50% cases and moderate expression in 25% cases. None of the adenoma cases showed strong AMACR expression.
Jiang Z et al., studied AMACR expression in adenomas, carcinomas and hyperplastic polyps [4]. They found positive AMACR expression in 79% (30/38) cases of adenoma. Lakis S et al., had confined their study to expression of AMACR in sporadic colorectal adenomas and they also found an increased expression of AMACR in adenomas associated with a higher grade of dysplasia, larger size and villous configuration [8].
Current study findings were similar to Jiang Z et al., and Lakis S et al., [4,8]. Since adenomas are precursors of colorectal carcinomas and they also show increased expression of AMACR, implies that AMACR plays a role in colorectal carcinogenesis. Also, this suggests possible role of AMACR as a diagnostic marker for neoplastic change.
AMACR expression in carcinomas
There were 44 cases of adenocarcinoma in the present study and 66% showed positive AMACR immunoreactivity. In adenocarcinoma, 6.81% showed a strong reaction for AMACR; 13.63% had moderate expression and 45.45% poor expression and 34.1% (15/44) showed no expression.
Overexpression of AMACR in colorectal carcinomas was reported by many workers. Studies by Jiang Z et al., showed increased expression in 69% (122/176) cases of colorectal carcinomas [4]. Shi X et al., found AMACR positivity in 59.4% (63/106) cases [7]. Lin A et al., performed their study on a tissue microarray with variable positive staining in 75% (123/163) cases and moderate to strong staining in 39% (63/163) cases [9]. Marx A et al., also performed their study on a tissue microarray but containing a much larger sample (1,315 cases of colorectal carcinomas) [10] compared to the study by Lin A et al., (163 cases of colorectal carcinomas) [9]. Findings of the present study corroborated with the previous works that expression of AMACR increases in carcinoma cases [Table/Fig-6].
Table showing the comparison of results obtained by various researchers.
| Authors | AMACR expression in adenomas | AMACR expression in carcinomas | Association with grade | Association of AMACR expression with stage | Association with nodal status |
|---|
| Jiang Z et al., [4] | Increased expression in 79% cases. | Increased expression in 69% cases | Significant association with grade | No correlation with Overall AJCC stage | No correlation with nodal status |
| Lin A et al., [9] | Increased expression in 64% cases. | Increased expression:Variable positive -75%, Moderate to Strong-39% | Significant correlation with grade | No correlation with AJCC staging | Significant association of AMACR expression and nodal status |
| Marx A et al., [10] | Adenomas not included in study. | Increased expression in 81.7% cases | Significant association with grade | Decreasing AMACR expression with higher tumour stage | |
| Present study | Increased expression in 75% cases | Increased expression in 66% cases | No association of expression in G1 and G2 | No association with AJCC stage | No association found |
None of the previous studies have compared the expression of AMACR in adenoma with carcinoma. In the present study, a comparison was made between AMACR expressions in adenoma (75% AMACR positivity) versus carcinoma cases (69% AMACR positivity). There was no significant difference of AMACR expression between adenoma and carcinoma cases. Therefore, AMACR cannot be used to distinguish between adenoma and carcinoma cases.
Association between AMACR expression and grades of adenocarcinoma
It has been found in previous studies that AMACR expression varies with the degree of differentiation of tumour. There is no or very low AMACR expression in cases of normal colon; significant up regulation in cases of adenomas as well as moderately differentiated adenocarcinoma. There is low expression in cases of poorly differentiated or undifferentiated carcinoma. Thus, AMACR expression is significantly associated with tumour differentiation and has a possible role in colorectal carcinogenesis. It has been hypothesized that overexpression of AMACR in the development of cancer may play an important role in providing energy for the neoplastic cells. In this hypothesis, as the tumours dedifferentiate, they take over other pathways to accomplish the same supply of energy as branched chain fatty acid oxidation. This phenomenon raises the possibility that during the progression of some carcinomas, a genetic abnormality may occur in a sub clone of cancer cells which eliminates the requirement for AMACR, although more direct evidence should be found to verify this [8].
Present study compared AMACR expression in Grade I-62% (13/21) cases and Grade II-71.4% (15/21) cases of adenocarcinoma to find out if there is any significant association between AMACR expression and grade of carcinoma. There were only two cases of Grade III (high grade) so they were not included in this analysis as such a small number is statistically insignificant.
In the study by Lin A et al., there was variable to strong positivity in 39% (63/163) cases and variable positivity in 75% (123/163) cases [9]. There was lack of staining in poorly differentiated carcinoma. They found significant correlation of AMACR expression with the grade of carcinoma. Marx A et al., also concluded that AMACR expression is significantly associated with grade of carcinoma [10].
Jiang Z et al., had 76% and 75% AMACR positivity in well and moderately differentiated cases [4]. This study also had comparable findings with 62% positivity in well differentiated and 71.4% in moderately differentiated. The difference was that they had 36% positivity in poorly differentiated cases and Grade III cases were not included in this analysis. Similarly Shi X et al., had compared well and moderately differentiated cases with poorly differentiated cases and got a significant difference of AMACR expression between the two groups [7].
Current study had only two cases of Grade III out of which one was negative and the other showed weak immunoreactivity for AMACR. The normal colonic tissue which were included as control tissue and also the normal colonic mucosa adjacent to the tumour showed weak or no positivity.
Association between AMACR expression and AJCC staging of colorectal carcinoma
Stage I and II had 68% (17/25) cases positive for AMACR and stage III had 63.15% (12/19) AMACR positivity. No significant association was found between AMACR expression and AJCC stage of colorectal carcinoma. The results of this study were similar to Jiang Z et al., and Lin A et al., [4,9]. Jiang Z et al., had found no correlation between AMACR expression and TNM staging or overall stage [4]. Lin A et al., did not find any correlation between AMACR expression and AJCC staging [9]. Marx A et al., had concluded that AMACR expression was significantly associated with tumour stage [10].
Association between AMACR expression and nodal status
No significant difference of AMACR expression was found between the node positive and node negative colorectal carcinomas. Present study results were similar to Jiang Z et al., who did not find any correlation between AMACR expression and nodal status [4]. On the contrary Lin A et al., found significant association between AMACR expression and nodal status [9]. They had 59% N0 cases showing low AMACR expression and 41% cases showing strong expression. In node positive group (N1 and N2) 65% low expression and 35% strong expression was seen.
Limitation
The major limitation of the current study is small sample size. Follow up of the cases is also necessary for correlating AMACR expression with disease free survival. Further, only one case of normal colonic mucosa was taken as control. A larger number of controls would be necessary to validate the findings.
Conclusion
This study shows an increased expression of AMACR in colorectal adenomas and carcinomas as compared to normal colonic epithelium thereby, suggesting a possible role of AMACR in colorectal carcinogenesis. However, it cannot be used to distinguish between adenomas and carcinomas. AMACR expression was not associated with tumour stage or nodal status. Further studies are required in this regard to establish AMACR as a prognostic marker in colorectal neoplasia and to find out its possible role in targeted therapy of the disease.