The strict control of blood glucose levels in adolescents with T1DM is accompanied by a considerable long term decrease in microvasular and macrovascular complications [1,2]. On the other hand, a significant number of people with T1DM, especially adolescents, could not achieve the vital goal of controlling blood sugar level, and they need larger amounts of insulin which has its unique side effects such as repeated episodes of hypoglycaemia, weight gain, dyslipidemia and so on [3,4].
Puberty goes with a considerable insulin resistance. This mainly affects peripheral glucose uptake with a small effect on fat metabolism [5,6]. Therefore, insulin dosage is inevitably increased in adolescents in order to overcome insulin resistance. Despite this, however, metabolic control often gets worse during the final stages of puberty [7].
Moreover, it has been observed that in adolescents with T1DM, the increase of growth hormone level during puberty is intensified compared with non-diabetic peers, leading to intensified insulin resistance in this population [8-14].
Therefore, there is a need for alternative or supplementary therapy strategies for adolescents with T1DM. One approach is to add other drugs to standard insulin therapy of T1DM in order to increase insulin sensitivity. Metformin, which is a biguanide works by inhibition of hepatic glucose production as well as increasing insulin-dependent peripheral glucose uptake [10].
It has been suggested that metformin decreases fatty acids as well as intestinal absorption of glucose; however, these effects are negligible compared with its comprehensive anti hyperglycaemic effect [7]. Some studies have investigated the effect of the addition of metformin to insulin on glycaemic control in patients with T1DM but their reports were different [15-24]. More studies are recommended to be conducted in order to confirm the metformin effect [6].
Since metformin as an adjunct therapy in adolescents with T1DM is not part of the routine protocol, we designed this study to show our experience on patients who referred to children’s medical center of Tehran University of Medical Sciences.
Materials and Methods
A quasi experimental study was designed after approval from the Ethical Committee of Tehran University of Medical Science, Tehran, Iran and and was recorded at IRCT Center (the code number IRCT2016 120110988N5).
The study population consisted of the patients aged over 10 with T1DM who were referred to Endocrinology Clinic of Children’s Medical Center of Tehran University of Medical Science in Iran. The sampling process continued from October 2013 to January 2014. All patients were followed for 12 months.
The sample size was obtained as follows: According to literatures, we estimated that metformin decreases HbA1c by 0.9% (μ1- μ0) with a standard deviation of 1.1 [7]. Thus, given α=0.05 and power (β) 80% for the study, the required sample size was obtained as 24. Considering a 20% probability for loss of follow up, the sample size was calculated as 30. The following formula was used, n = {(Z (1-α/2) +Z (1-β))2 × s.d2}/(μ1- μ0)2.
This study tried to prevent loss of follow up as much as possible by selecting samples following an accurate check up of their important organs such as kidney and liver, training cases to prevent problems such as Diabetic Ketoacidosis (DKA) and hypoglycaemia, performing periodical visits on a regular basis and establishing phone communication with the cases for investigating and controlling purposes.
Inclusion criteria included age 10-17 years T1DM diagnosed by the first presentation in diabetic ketoacidosis (blood glucose > 200 mg/dl, venous pH < 7.3, serum bicarbonate level < 15 mEq/l and ketones in urine) with the history of polyuria, polydipsia, weight loss and without previous obesity, overweight and no family history of diabetes [25]; duration of T1DM > one year; insulin dosage > one unit / kg / day (NPH plus regular, multiple injection) and HbA1c > 8% in the past six months as suboptimal metabolic control [7,10]. Exclusion criteria included nephropathy (albumin excretion rate 200 g/min); proliferative retinopathy; occurrence of DKA more than twice per year; recurrent severe hypoglycaemia (with loss of consciousness requiring assistance to treat, more than two episodes in the past year); renal or hepatic dysfunction; another serious medical illness and the presence of a known eating disorder.
The patients were included in the study after collecting their parent’s written consent. The studied variables included HbA1c, the average dosage of insulin, Body Mass Index (BMI), serum Glutamic Oxaloacetic Transaminase (SGOT), serum Glutamic Pyruvic Transaminase (SGPT), serum creatinine, lactate, High-density lipoprotein (HDL), Low-density lipoprotein (LDL), triglyceride and cholesterol levels.
HbA1c was measured in the beginning of the study and repeated with three months intervals till the end of it. The other variables were measured twice; in the beginning of the study and at the end of it (after 12 months).
In those matching the inclusion criteria, metformin tablet (MINOO-METFORMIN 500 mg TAB) was added to patient’s insulin therapy with the following dosages:
weight < 50 kg: starting with 500 mg/day and then increasing gradually to 1000 mg/day;
weight more than 50 kg and <75 kg: starting with 500 mg/day and then increasing gradually to 1500 mg/day;
Weight>75kg: starting with 500 mg/day and then increasing gradually to 2000 mg/day.
The above dosages were prescribed in three equally divided doses along with meals. Metformin was administered for 12 months.
In every checkup it was made sure that the cases had used the acurrate doses of medication (insulin and metformin).
Statistical Analysis
Data was analysed by SPSS (version 18) software. Frequency and frequency percentage were calculated for qualitative variables, and mean and standard deviation were calculated for quantitative variables. Kolmogrov-Smirnov test was used to investigate the normal distribution of the variables. Paired t-test and Repeated Measure ANOVA were used to examine the study’s hypothesis. For the statistical analysis, p-value <0.05 was considered as significant.
Results
Overall 39 patients were investigated based on inclusion criteria, 10 out of them were excluded (two with history of occurrence of DKA more than twice per year, two with history of acute hypoglycaemia with loss of consciousness to a level necessitating help more than twice per year, three had underlying diseases, and three patients’ parents did not give consent for participation in the study). Finally, data from 29 patients was analysed.
Of all the cases, 17 were female (58.6%) and 12 were male (41.4%). The age of cases ranged from 11 to 17 years with a mean of 13.4±1.5 year. Diabetes duration was 5.9±2.2 years (1.5-10 years). 10 (34.5%) were in tanner 3, 7 (24.1%) were in tanner 4 and 12 (41.4%) were in tanner 5.
[Table/Fig-1] compares the values of weight, BMI, insulin dosage, serum creatinine level, lactate, SGOT, SGPT, triglyceride, cholesterol, LDL and HDL in the beginning and at the end of the study.
Comparing the level of outcome variables before and after the addition of metformin tablet to patient’s insulin therapy.
| Variable | Before | After | p-value |
|---|
| Weight (kg) | 59.8±7.9 | 66.2±8.6 | < 0.001* |
| BMI (kg/m2) | 24.9±2.5 | 25.7±2.4 | 0.007* |
| Dosage of insulin (u/kg/d) | 1.1±0.2 | 0.9±0.2 | <0.001* |
| Serum Creatinine (mg/dl) | 0.75±0.09 | 0.78±0.08 | 0.1 |
| Serum Lactate (mg/dl) | 10.1±3.1 | 9.4±3.4 | 0.12 |
| Serum SGOT (mg/dl) | 24.1±4.6 | 23±3.6 | 0.28 |
| Serum SGPT (mg/dl) | 25.7±4.1 | 25.4±3.7 | 0.7 |
| Serum TG (mg/dl) | 154.8±61.1 | 146.8±54.7 | 0.1 |
| Serum Cholesterol (mg/dl) | 191.7±54.3 | 186.3±51.7 | 0.06 |
| Serum LDL (mg/dl) | 95.7±22.8 | 94.8±30.1 | 0.7 |
| Serum HDL (mg/dl) | 50.4±6.5 | 52.6±5.2 | 0.7 |
Paired- t-test was used for analysis.
*p-value <0.05 considered to be significant.
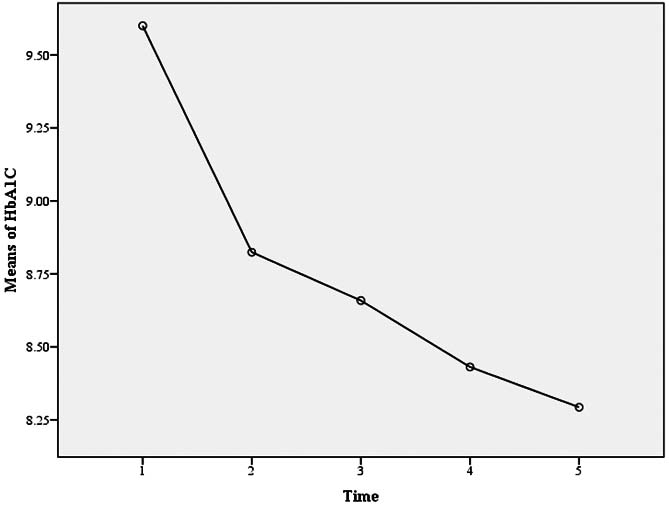
During the study, HbA1c level was registered for five times. The values were 9.6±1.3, 8.8±0.9, 8.6±1.1, 8.4±0.8, and 8.2±0.8 in the beginning of the study, in the second, third, fourth measurement, and at the end of the study. As can be seen in [Table/Fig-2], HbA1c level was significantly reduced during the study (p<0.001).
The HbA1c levels that was registered in the beginning of the study, in the second, third, fourth measurement, and at the end of the study
During the study, metformin did not increase, lactate, AST, and ALT [Table/Fig-1]. The increasing of serum creatinine was not significant (p= 0.1). Moreover, no hypoglycaemia was reported.
Discussion
In this study, the effect of metformin as an adjunct therapy, on glycaemic control in the adolescents with T1DM was investigated.
Those oral medications used for the treatment of Type 2 diabetes could be a helpful supplementary therapy for individuals with T1DM and insulin resistance. Among biguanides, metformin primarily reduces hepatic glucose output; however, it can also affect insulin sensitivity, especially in combination with insulin. Both mechanisms could be helpful for individuals with T1DM and insulin resistance [10].
Studies showed the average of HbA1c levels in adolescents with T1DM is 1% higher than in adults, despite receiving more insulin. The proposed mechanism is elevated levels of growth hormone (anti-insulin hormones) and sex steroids, during the period of active pubertal maturation [15].
The present study showed that adjunctive metformin therapy significantly reduced HbA1c during the study and following a 12 months period.
Various studies of adjunctive metformin therapy in children and adult have reported different results for HbA1c values [7,10,15-24].
In a systematic review by Abdelghaffar S and Attia AM, some evidence suggested that metformin treatment lowered glycosylated HbA1c in adolescents with Type 1 diabetes and poor metabolic control and stronger evidence is required from larger studies to document the long term effects on metabolic control in those patients [6].
Hamilton J et al., examined the addition of metformin to insulin and standard diabetes management in 27 adolescents with T1DM in a placebo-controlled 3-month trial and reported metformin treatment significantly reduced (HbA1c was 0.6% lower in the metformin group than in the placebo group) [10].
Moreover, study done by Särnblad S on 26 adolescents with T1DM showed that HbA1c level was significantly decreased by 0.9% in the group receiving metformin [7].
Study done by Urakami T showed metformin decresed HbA1c in nine adolescents and young adults with T1DM [16]. Also the study of Gomez R et al., showed effect of metformin in reduction of HbA1c in 10 adolescents and young adults with T1DM [17].
The study of Khan AS et al., also showed the effect of metformin on the reduction of HbA1c level in overweight adults with T1DM [18].
In contrast some studies have reported no significant reduction in HbA1c. Vella S et al., reported in a meta-analysis on the use of adjunctive metformin therapy in children and adults with T1DM that metformin had not effect on significant reduction in HbA1c [1].
Codner E et al., investigated the effect of metformin (850 mg bid) on hyperandrogenism and glycaemic options in female adolescents with T1DM and found no differences in HbA1c and the insulin dosage between the placebo and metformin group [19].
Study done by Libman IM et al., showed among overweight adolescents with T1DM, the adjunctive metformin therapy did not significantly reduce HbA1c after six months [20]. Studies done by Nadeau KJ and Nwosu BU showed no significant change in HbA1c by 6-9 months adjunctive metformin therapy in adolescents with T1DM [5,15].
Also the studies of Meyer L, Moon RJ and Jacobsen IB showed no change in the HbA1c in adults with T1DM receiving metformin [21,22,24].
In the present study, the insulin dosage received by the cases was significantly reduced at the end of the study. This finding is also consistent with Hamilton’s study that found daily insulin dosages were significantly lower in metformin group [10]. Urakami T et al., also showed insulin requirement decreased significantly after the start of metformin therapy [16]. Studies done by Meyer L and Khan AS also showed the addition of metformin can reduce the total daily insulin dose in adult with T1DM [18,21].
However, study done by Nwosu BU and Särnblad S showed no significant change in the dosage of insulin in the adolescents who received metformin [7,15].
Weight and BMI were increased in our cases. This disagrees with the findings of Hamilton J, Lund SS, and Jacobsen IB. Although in study done by Hamilton J, the weight loss of the cases who received metformin was not significant compared with control group [10,23,24]. Study done by Moon RJ showed that the cases who continued metformin, experienced BMI loss following a two-year period [22].
Serum lipid of our cases was decreased but it was not statistically significant. Study done by Meyer L showed a significant decrease in total cholesterol as well as LDL [21].
In this study, the insulin sensitivity was not measured directly. However, the evidence indicating the decrease of HbA1c as well as insulin level could be considered as an indicator for the improvement of insulin sensitivity in cases who receive metformin.
The present study’s strength was the long duration of intervention and follow up of patients (12 months).
Limitation
Limitation of our study was that we did not have a control group. We used before and after study design because there are many confounding factors such as socioeconomic status, lifestyle, diet, education, patient compliance in the control of diabetes and in our cases parental education and parental involvement in diabetes management [15]. Although, the necessary training about lifestyle was given to patients and parents, but intake of calories and physical activity level was not monitored and analysed.
Conclusion
Adjunctive metformin therapy reduced HbA1c value and the insulin dosage received in adolescents with T1DM in our study. Conducting more long term studies, preferably multicentral studies with control group, is recommended in order to evaluate if the effect of metformin on metabolism control is sustained and whether there are benefits for cardiovascular outcomes in patients with T1DM.
Paired- t-test was used for analysis.
*p-value <0.05 considered to be significant.