Obesity is an emerging public health problem in the Indian population in young adults and its prevalence in developing countries has risen to epidemic proportions. Obesity increases morbidity and mortality due to many chronic health ailments, such as cardiovascular disease, type 2 diabetes, dyslipidaemia and fatty liver disease [1]. Less is known regarding influence of central obesity on respiratory functions. The concept of lung age relates a person’s current lung function at which his/her lung function would be considered abnormal. Thus, an elevated lung function indicates as if the lungs have aged beyond the individual’s chronological age. The concept of lung age was introduced by Morris JF and Temple W [2].
Body Mass Index (BMI) is for general obesity and is not an ideal measure for excess body weight as a predictor of pulmonary function compared with WC [3]. Despite its acknowledged importance, no unified definition exists for central obesity; several anthropometric indexes such as WC, Waist-Hip Ratio (WHR), index of central obesity, CI etc., are being used. CI is a simple method to assess abdominal obesity and its association with cardiovascular risk factors [4].
An individual with CI of 1.25 indicates that he/she has a WC 1.25 times larger than the circumference of a cylinder with height and weight of that person. The actual range of CI is 1.00-1.73. Greater conicity in Asian young adults of both sexes, than in Europeans, was observed in the top tertiles of weight and BMI [5]. CI has advantages over WHR such as, it has got a theoretical range, built in adjustment of WC for height and weight and does not require hip circumference to measure fat distribution [4].
Much less is the fact that influence of central obesity is linked with lung age in young adults. Thus, the aim of this study was to determine central obesity by CI and its influence on lung age in young adults.
Materials and Methods
Sample size: Sample size was estimated based on the prevalence of central obesity at 73%. By using the formula = (1.96)2pq/d2. n= 287 at 10% error and 95% Confidence interval.
The final adjusted sample size, allowing non response rate of 10% in, the adjusted sample size was n = 287/0.90 = 319 subjects.
A total of 319 young adults in the age group 18-25 years were recruited for this cross-sectional observational study done during the period Dec 2015-June 2016. Written informed consent and IEC was obtained. The subjects were recruited from in and around Kolar district, Karnataka, India who had sedentary lifestyle.
Anthropometric parameters like BMI, WC, height, and weight was measured. Weight and height was measured with minimal attire. BMI was calculated by dividing weight (kg) with the square of height (m2). WC (m) was taken horizontally within 1 mm, using plastic tape measuring at mid point between costal margin and iliac crest in the mid axillary line with the subject standing and at the end of gentle expiration. CI was calculated using the following formula:
CI= WC (m)/ [0.109 X√ {Bodyweight (kg)/ Height (m)}].
where 0.109 is a constant which results from the conversion of units of volume and mass into units of length [6].
Based on CI, they were classified into obese and non obese. For males 1.25 and for females 1.18 cut offs were used to classify CI into normal and high categories [6].
Prerequisites for spirometry: History regarding exercise within the preceding hour was enquired and that individual was requested to avoid exercise for 24 hours. Before starting the lung function tests, the subjects were asked to loosen their tight clothing. The study was conducted at the same time for all the days to rule out diurnal variations. Spirometry was performed using the instrument SPIROTECH and was carried out after demonstrating the procedure to the participants. The subjects were asked to take deep inspiration from the external air followed by forceful expiration into the mouthpiece of the SPIROTECH in a standing posture. It was ensured that the mouthpiece was inserted without any leakage of air or obstruction by the lips or teeth and forced expiration continued to completion without a pause. The subjects inspired rapidly again to maximum capacity. The subjects were asked to repeat the procedure three times and the best one was taken. All the lung volumes and capacities obtained (FVC, FEV1, PEFR and FEV1/FVC ratio, lung-age, forced expiratory flow from 25-75% of vital capacity {FEF25-75%} were expressed with correction for Body Temperature at the Ambient Pressure, Saturated with Water Vapour (BTPS). The lung age difference was estimated by subtracting lung age obtained by Pulmonary Function Test (PFT) with chronological age. The subjects who were smokers or suffering from neuromuscular disorders, kyphoscoliosis and with any respiratory illness were excluded from the study [7].
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) version 17.0 was used for the statistical analysis. The data were presented as a mean±standard deviation. Independent t-test was applied to compare the measured general characteristics and pulmonary function values between obese and non obese young adults. Paired t-test was applied between chronological age and lung age in obese and non obese young adults. Pearson’s correlation test was used to determine the relationship between CI and pulmonary function variables. Chi-square test was used for analysing categorical variables. Level of significance was set at p<0.05.
Results
Values are expressed as Mean±SD. The p-value is of independent t-test where p<0.05 is significant.
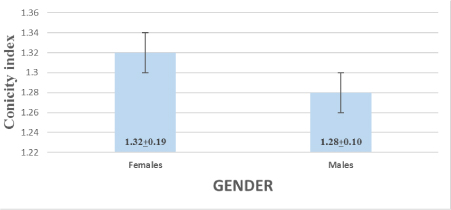
[Table/Fig-1] shows a significant increase in mean values of CI between females and males (t=2.34, p<0.02).
Mean of CI in males and females.
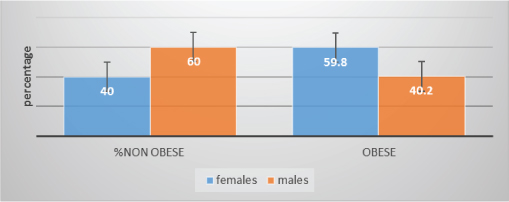
[Table/Fig-2] shows χ2 of 9.52 with 2df and p<0.002 with 95% CI females are obese compared to males assessed by CI. Females were more obese compared to males.
Percentage of obese and non obese, according to gender, determined by CI.
In total 319 subjects were enrolled; 80 (25%) were non obese and 239 (75%) were obese. Comparison of the mean values of the anthropometric and pulmonary function parameters between obese and non obese individuals are shown in [Table/Fig-3]. There was a significant increase (p<0.05) in the mean values of CI, lung age and lung age difference in obese young adults compared to non obese. The other PFT parameters i.e., FVC, FEV1, PEFR, FEF 25-75% showed significant decrease in obese young adults compared to non obese.
Independent t-test comparing anthropometric and pulmonary function test in obese and non obese young adults obtained by CI (males<1.25, females <1.18 are non obese).
| Parameter’s | Non obese (n=80)Mean+SD | Obese (n=239)Mean+SD | t-value | p-value |
|---|
| Age (years) | 19.25±1.30 | 19.33±1.65 | -0.44 | 0.657 |
| BMI (kg/m2) | 23.19±3.89 | 23.28±5.35 | -0.15 | 0.880 |
| WC (m) | 0.78±0.10 | 0.90±0.13 | -8.3 | 0.001* |
| Conicity index | 1.16±0.08 | 1.36±0.15 | -14.81 | 0.001 |
| FVC (l) | 2.64±0.67 | 2.37±0.62 | 3.13 | 0.002 |
| FEV1 (l) | 2.55±0.62 | 2.31±0.59 | 3.08 | 0.002 |
| FEV1/FVC % | 96.89±4.31 | 97.06±4.09 | -0.30 | 0.760 |
| FEF25-75 (l/s) | 5.27±8.70 | 3.78±1.09 | 1.53 | 0.010 |
| PEFR (l/s) | 5.71±1.85 | 4.22±0.97 | 6.86 | 0.001 |
| Lung age (years) | 21.30±2.64 | 23.87±3.03 | -7.2 | 0.001* |
| Lung age difference (years) | 2.05±2.75 | 4.54±3.20 | -6.67 | 0.001* |
* Significant
In [Table/Fig-4], paired t-test was applied between chronological age and lung age in obese and non obese young adults based on CI, which showed significant increase in lung age in both groups.
Paired t-test of chronological age and lung age in obese and non obese young adults based on conicity index.
| Groups | Chronological age(years) | Lung age (years) | t-value | p-value |
|---|
| Non obese | 19.25±1.30 | 21.30±2.64 | -6.66 | 0.001* |
| Obese | 19.33±1.65 | 23.87±3.03 | -21.50 | 0.001* |
* Significant
[Table/Fig-5] shows that there was a weak positive correlation between CI and lung age (years) in obese group but statistically non significant. In non obese young adults, the lung-age showed negative weak correlation. FEV1/FVC% showed weak positive correlation (r=0.020) in obese young adults compared to non obese.
Pearson’s correlation between CI and anthropometric, pulmonary function parameter’s in non obese and obese young adults.
| Parameters | Non obese | Obese |
|---|
| r | p | r | p |
|---|
| WC (m) | 0.506 | 0.001* | 0.613 | 0.001* |
| BMI (kg/m2) | 0.090 | 0.426 | -0.081 | 0.214 |
| FVC (l) | 0.217 | 0.053 | -0.072 | 0.266 |
| FEV1 (l) | 0.182 | 0.105 | -0.601 | 0.359 |
| FEV1/FVC% | -0.262 | 0.019* | 0.020 | 0.760 |
| FEF25-75 (l/s) | 0.048 | 0.675 | 0.005 | 0.935 |
| PEFR (l/s) | 0.090 | 0.426 | 0.013 | 0.841 |
| Lung age (years) | -0.023 | 0.841 | 0.098 | 0.129 |
* Significant
[Table/Fig-6] shows that FEV1/FVC% exhibited a significant positive correlation in obese young adults compared to non obese.
Pearson’s correlation between lung age difference and other pulmonary function parameters in non obese and obese young adults obtained by CI.
| Parameters | Non obese | Obese |
|---|
| r | p | r | p |
|---|
| FVC(l) | -0.275 | 0.013* | -0.126 | 0.052 |
| FEV1(l) | -0.307 | 0.006* | -0.149 | 0.021* |
| FEV1/FVC% | -0.066 | 0.561 | 0.141 | 0.030* |
| EFR(l/s) | 0.165 | 0.144 | 0.083 | 0.20 |
* Significant
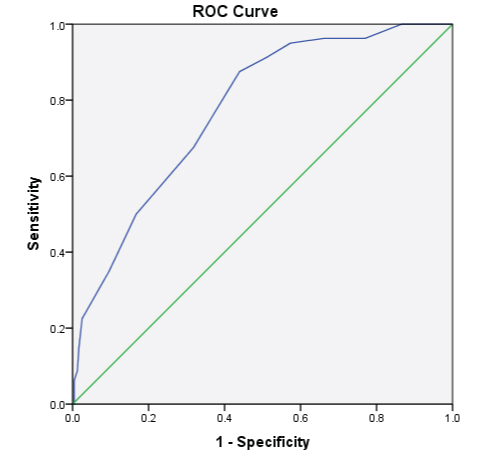
[Table/Fig-7] ROC analysis of lung age (years) in obese and non obese young adults assessed by CI showed an accuracy of 77.5% (AUC-0.775 at 95% CI-0.720 to 0.830) which is statistically significant (p<0.001). The optimal cut off value to discriminate lung age (years) in young individuals in the age group of 18-25 years was 23.5 years, the sensitivity being 87.5% and specificity being 43.9%.
ROC analysis of lungage (years) in obese and non obese young adults assessed by CI.
Discussion
The present study was conducted to assess the effect of central obesity on lung age in young adults. This study was carried out in 319 young individuals whose age was matched in obese (75%) and non obese (25%) young adults and obesity criteria was taken based on their CI. Females (59.8%) were significantly more obese compared to males (40.2%). The mean CI in females is significantly increased (p<0.05) compared to males which is consistent with other studies [8].
In our study, central obesity, assessed by CI was significantly increased in obese compared to non obese individuals. Its rationale is to quantify the excess abdominal fat for a person with a prescribed WC in relation to the circumference of the cylinder generated with that persons weight and height based on a constant body density. CI has a theoretical range 1.0< CI<1.73 representing perfect cylindrical and perfect biconal body shapes, respectively.
In the present study, CI in obese was towards biconal body shape indicating central obesity. The CI estimates fat accumulation in the abdomen which leads to a deviation of body shape from a cylindrical to a double-cone shape, i.e., two cones with a common base at the waist level [6]. This abnormal deposition of fat in the abdomen has been described as the type of obesity that greatly threatens the individual’s health leading to metabolic derangements, coronary heart disease, and poor lung function [9,10]. In our study, the BMI was found to be non significant and an increase in CI in the presence of normal BMI was observed. Few studies indicate that measures of central obesity are better discriminators of morbidity compared to BMI. As opposed to BMI, which does not differentiate between muscle and fat, CI may be a useful tool to identify individuals with central obesity, but who are not necessarily obese or overweight.
Based on CI, no studies have been done to assess the influence of central obesity on lung functions, particularly lung age. There is significant increase in mean values of lung age and lung age difference in obese young adults compared to non obese. The other PFT parameters FVC, FEV1, PEFR, FEF 25-75%, showed significant difference in obese young adults compared to non obese (p<0.05) [Table/Fig-3]. There was significant increase in lung age compared to the chronological age in both the groups [Table/Fig-4].
The idea of “lung age” was developed as a way of making spirometric parameters easier to understand and also as a potential psychological tool to show an individual who is at risk of apparent premature ageing of their lungs. Increased lung age in obese young adults is a clear message that the lungs are undergoing accelerated deterioration and a way of expressing lung damage rather than using mathematical concepts of a percentage of the expected value of FEV1 for height, age and gender [11]. The optimal cut off value to discriminate lung age in young adults who are obese and non obese in the chronological age group of 18-25 years is 23.5 years [Table/Fig-7].
In the present study, spirometric variables such as FEV1 and FVC has decreased with increased CI; however the effect is small and both FEV1 and FVC were usually within normal range in healthy obese young adults. FEV1/FVC was increased and showed a weak positive correlation in obese young adults, indicating both FEV1 and FVC are affected to same extent. PEFR was significantly decreased in obese young adults, which indicates decrease in the strength of the expiratory muscles generating the force of contraction, the elastic recoil pressure of the lungs and the airway size [12]. In our study, correlation between lung age difference and FEV1/FVC showed a significant positive correlation in obese young adults indicating mild restrictive lung disorder [Table/Fig-6].
Central obesity affects respiratory functions by various mechanics. Central obesity in particular compromises lung mechanics by restricting lung volumes, reducing chest wall compliance, and attenuating respiratory muscle efficiency [12]. Central accumulation of fat mechanically affects the expansion of the diaphragm or impedes the descent of the diaphragm during forced inspiration. The published literature mostly indicates that abdominal fat alters the pulmonary mechanics, causing restriction during breathing, potentially reducing respiratory volumes mainly FEV1 and FVC. Alteration in mechanical effect, decrease in compliance and increase in the resistance of the respiratory system is more prominent in central obesity than overall body fat distribution leading to increased respiratory demand.
CI shows strong positive correlation with WC (r=0.613, p<0.001) in obese individuals. [Table/Fig-5] indicating that WC has an strong effect on the diaphragm by limiting its movements.
Few imaging studies reveal abnormality in regional ventilation in obese individuals. Normally in an upright non obese individual the distribution of regional ventilation is greatest in the lower, and decreases towards the upper zones. In obese individuals, this distribution may be reversed so that ventilation is preferentially distributed in the upper zones of the lung, leaving lower dependent zones relatively under-ventilated [13]. In addition, visceral adipose tissue influences circulating concentrations of Interleukin-6 (IL-6), Tumour Necrosis Factor α (TNF-α), leptin, and adiponectin, which are cytokines that may act through systemic inflammation, thus, negatively affecting pulmonary function [14-17]. Investigators have reported an indirect association of serum leptin concentrations with FEV1, as well as increased levels of C-Reactive Protein (CRP), leucocytes, and fibrinogen, which are markers of systemic inflammation. This indicates that inflammation may be the link between visceral obesity and pulmonary function [18].
Limitation
The limitation of this study is that the obesity in younger individuals, which is influenced by physical activity, lifestyle modification and dietary habits, has not been assessed which might influence the pulmonary function.
Conclusion
CI can be used as a new index of anthropometric measure to assess central obesity, than BMI which might not be a reliable anthropometric measure as it indicates overall body fat distribution and muscle mass. The present study shows that CI is increased in obese though their BMI is normal. Thus, an individual can calculate CI and see whether obesity is towards cylindrical or biconal shape and take appropriate measures to reduce weight.
In our study, spirometry results in young adults expressed as “lung age” gives a clear message that increase in lung-age indicates that lungs are undergoing a gradual deterioration. Thus, it can be slowed if they take proper measures to reduce obesity which influences pulmonary function. So, lung age can be used as a psychological tool to explain to the person how his/her lungs are deteriorating in a simple way, instead of explaining the individual the mathematical equation of % predicted FEV1 or FEV1/FVC ratio.
* Significant
* Significant
* Significant
* Significant