Pemphigus is a group of chronic Autoimmune Blistering Disorders (AIBD) characterized clinically by flaccid bullae and mucocutaneous erosions and histopathologically by intraepidermal acantholysis. The various types of pemphigus include pemphigus vulgaris and its variant pemphigus vegetans, pemphigus foliaceous and its variants pemphigus erythematosus and pemphigus herpetiformis, IgA pemphigus, paraneoplastic pemphigus and drug induced pemphigus. Of these, pemphigus vulgaris constituting 75 to 92% [1], was associated with high mortality before the usage of corticosteroids. DCP therapy, introduced in 1982 and widely used since 1984, consists of monthly pulses of supra pharmacologic doses of dexamethasone and cyclophosphamide [2]. Recently biological therapies targeting autoreactive B cells have been tried in pemphigus with good results. Rituximab is a murine human chimeric monoclonal antibody targeted against CD20. It acts by destruction of autoreactive B cells, its effect lasting six to nine months. Sustained remission after repopulation of B cells has been documented [3]. This study aims to assess the effectiveness of rituximab and confirm the effectiveness of the rheumatoid arthritis protocol in patients with pemphigus vulgaris, to study the relationship between various patient factors, disease factors and clinical end points, to compare early and late end points after rituximab with those of conventional treatment and to identify the common adverse effects of rituximab.
Materials and Methods
It was an open label prospective interventional study conducted from September 2013 to May 2015 in the Department of Dermatology, Stanley Medical College, Chennai, Tamil Nadu, India after obtaining institutional ethical committee clearance and written informed consent from the patients included in the study. Due to rarity of the disease all the patients with pemphigus vulgaris who attended the out patient department, satisfying exclusion and inclusion criteria and willing to participate in the study, were included. Ten were found to be refractory to conventional therapy and 10 were new cases. The inclusion criteria were proven cases of pemphigus vulgaris in persons above18 years of age. The exclusion criteria were active or latent tuberculosis, HIV 1 or HIV 2 positivity, active or chronic Hepatitis B, Hepatitis C, coexistent pulmonary, renal, gastrointestinal and other disseminated infections, extensive wound infections, septicaemia, pregnancy, lactation, cardiac disease (New York Heart Association class IV) and history of bronchospasm, angioedema. The early end points of the study were time taken to attain control of disease activity and duration of consolidation phase; the late endpoint was duration of complete remission off treatment. The endpoints were in accordance with the consensus statement on definitions of disease end points and therapeutic response for pemphigus [4].
The age of the patient, sex, duration of disease and a detailed history were recorded. The extent of skin/mucosal involvement was clinically assessed, baseline Pemphigus Activity Score (PAS) [5] was calculated and the diagnosis was confirmed clinically and histopathologically. Selected patients were evaluated for baseline parameters such as complete blood count, renal and liver function tests. Guidelines for vaccination were adhered to as per recommendations for immunisation in patients undergoing planned immunosuppression [6].
Rituximab was given according to Rheumatoid Arthritis protocol, where one gram administered as intravenous infusion at two weeks interval. This protocol was chosen as it was found to have higher response rate, lower mortality and lacked non responders [7]. The new cases received rituximab with adjuvant daily systemic corticosteroids and immunosuppressants which were tapered later. In patients already on steroids or immunosuppressants, this was continued and withdrawn slowly during the consolidation phase. Once the general condition stabilized, the patient was discharged and reviewed as outpatient after two weeks, and every month thereafter. During each follow up, patients were examined for the status of old lesions, appearance of new lesions, PAS, signs of infection; complete blood count, renal and liver function tests were done, early and late end points and adverse events present were noted.
In all the patients, the disease parameters were compared before and after rituximab infusion. In those patients refractory to conventional therapy, details of early and late endpoints after conventional treatment were noted from old records and were compared with the end points after they received rituximab infusion. The end points after rituximab in patients who received rituximab as first line therapy were compared with the endpoints after rituximab in refractory patients. Finally, the disease parameters and end points were compared between those who relapsed and those who remained in remission. Data was analysed using SPSS windows software version 17.0.
Results
The mean age of the study group was 41.35 years. There were 14 females (70%) and six males (30%). Disease duration ranged from 1-36 months, the mean duration being 11.7 months. Follow up period varied from three to 23 months. The mean PAS score at baseline was 7, at one month post rituximab was 1.95 and at three months post rituximab 0.66. After rituximab, disease control was attained within 2-16 weeks with a mean time of 3.65 weeks. Consolidation phase ranged from 1- 3 months, the mean duration was 1.6 months. Remission period ranged from 3 -22 months, the mean duration of remission was 11.45 months [Table/Fig-1]. Thirteen patients remained in remission for varying periods of 3-22 months. This variation probably reflects the difference in the follow up period for each patient. Seven (35%) patients relapsed during the study period, two at six months, three patients at 10 months and two at 16 months after rituximab.
Tabulation of age, gender, baseline PAS, duration of disease, early and late end points with DCP and Rituximab.
| S.No | Age | Sex | Duration of Disease in Months | PAS* - Total | Duration of Follow Up In Months | PAS at one Month | PAS at three Months | Time Taken to Attain Disease Control in Weeks (Rituximab) | Duration of Consolidation Phase (Rituximab) in Months | Duration of Remission Off Treatment (Rituximab) | Time Taken to Attain Disease Control in Weeks (DCP) | Duration of Consolid-ation Phase In Months (DCP) | Duration of Remission Off Treatment (DCP) | Relapse = 1, Remission = 0 |
|---|
| 1. | 38 | F | 24 | 8 | 23 | 4 | 0 | 4 | 2 | 9 | 12 | 5 | 4 | 1 |
| 2. | 44 | F | 24 | 6 | 22 | 4 | 4 | 12 | 3 | 6 | 16 | 5 | 4 | 1 |
| 3. | 55 | F | 10 | 7 | 22 | 0 | 0 | 2 | 1 | 22 | 12 | 7 | 0 | 0 |
| 4. | 26 | M | 2 | 7 | 22 | 2 | 0 | 2 | 2 | 22 | | | | 0 |
| 5. | 43 | M | 12 | 7 | 21 | 3 | 2 | 4 | 2 | 10 | 12 | 0 | 0 | 1 |
| 6. | 48 | M | 3 | 8 | 21 | 2 | 0 | 2 | 1 | 21 | | | | 0 |
| 7. | 30 | F | 24 | 7 | 18 | 3 | 1 | 2 | 2 | 14 | 12 | 8 | 4 | 1 |
| 8. | 60 | F | 1 | 4 | 18 | 2 | 0 | 2 | 1 | 16 | | | | 1 |
| 9. | 20 | F | 3 | 8 | 16 | 3 | 0 | 3 | 2 | 16 | | | | 0 |
| 10. | 40 | M | 6 | 7 | 16 | 0 | 0 | 2 | 1 | 16 | | | | 0 |
| 11. | 36 | F | 4 | 6 | 14 | 0 | 0 | 2 | 1 | 14 | | | | 0 |
| 12. | 50 | M | 36 | 8 | 11 | 5 | 4 | 16 | 3 | 6 | 16 | 6 | 2 | 1 |
| 13. | 39 | F | 30 | 8 | 11 | 3 | 0 | 4 | 2 | 9 | 20 | 10 | 4 | 1 |
| 14. | 41 | F | 10 | 7 | 11 | 3 | 0 | 3 | 1 | 11 | 12 | 7 | 0 | 0 |
| 15. | 32 | F | 10 | 8 | 9 | 2 | 0 | 3 | 1 | 9 | 8 | 8 | 0 | 0 |
| 16. | 35 | F | 2 | 6 | 9 | 0 | 0 | 2 | 1 | 9 | | | | 0 |
| 17. | 44 | F | 1 | 7 | 6 | 2 | 0 | 2 | 2 | 6 | | | | 0 |
| 18. | 50 | F | 9 | 7 | 6 | 0 | 0 | 2 | 1 | 6 | 16 | 5 | 0 | 0 |
| 19. | 72 | M | 3 | 7 | 4 | 0 | 0 | 2 | 1 | 4 | | | | 0 |
| 20. | 24 | F | 20 | 7 | 3 | 2 | 0 | 2 | 2 | 3 | | | | 0 |
| MEAN | 41.35 | | 11.7 | 7 | 14.25 | 1.95 | 0.66 | 3.6 weeks | 1.6 months | 11.45 months | | | | |
*- Pemphigus Activity Score.
Paired samples t-test was used to analyse the differences between PAS - base line and PAS -one month, PAS - base line and PAS -three month and PAS-one month and PAS -three month [Table/Fig-2]. Rituximab has a significant effect in decreasing PAS and reducing disease activity at one month and at three months post infusion. As most of the patients had been withdrawn from steroids and immunosupressants during the consolidation phase, the significant reduction in PAS at three months from PAS at one month can be attributed to rituximab alone. A Pearson correlation computation revealed no correlation between age or extent of disease and reduction in PAS at one and three months. There was a significant positive correlation between duration of disease and time taken to attain disease control/duration of consolidation phase. There was a negative correlation, though not statistically significant, between duration of disease and duration of remission off treatment. This may be because of the difference in the follow up period.
Comparing disease parameters before and after Rituximab.
| Paired Samples | Paired Differences | t | df$ | Significance(Sig) (2 tailed) |
|---|
| MEAN | S.D* | S.E.M* |
|---|
| Pair 1: PAS Baseline Vs PAS one Month | 5.000 | 1.556 | 0.348 | 14.371 | 19 | 0.000 |
| Pair 2: PAS Baseline Vs PAS three Months | 6.450 | 1.605 | 0.359 | 17.971 | 19 | 0.000 |
| Pair 3: PAS one Month Vs PAS three Months | 1.450 | 1.276 | 0.285 | 5.081 | 19 | 0.000 |
*- Standard Deviation; * - Standard Error Mean; $ - Degree of freedom
A paired samples t-test was computed to assess the relationship between early and late end points with DCP and Rituximab for the same patient [Table/Fig-3]. There was a significant difference in the early and late end points attained with DCP and rituximab. With rituximab, time taken to attain disease control and consolidation phases was shorter and remission was prolonged.
Comparing the end points after DCP and after rituximab in patients who received both.
| Paired Samples | MEAN | N | S.D | S.Error Mean(S.E.M) | Sig | PAIRED DIFFERENCES | t | df | Sig(2 tailed) |
|---|
| MEAN | S.D | S.E.M |
|---|
| Time taken to attain disease control (weeks) | Rituximab | 5.20 | 10 | 4.803 | 1.519 | 0.266 | -8.40 | 4.671 | 1.477 | -5.686 | 9 | 0.000 |
| DCP | 13.60 | 10 | 3.373 | 1.067 |
| Duration of consolidation phase (months) | Rituximab | 1.78 | 9 | 0.833 | 0.278 | 0.581 | -5.00 | 2.052 | 0.087 | -7.276 | 8 | 0.000 |
| DCP | 6.78 | 9 | 1.716 | 0.572 |
| Duration of remission off treatment (weeks) | Rituximab | 8.80 | 5 | 3.271 | 1.463 | 0.415 | 5.200 | 2.950 | 1.319 | 3.942 | 4 | 0.017 |
| DCP | 3.60 | 5 | 0.894 | 0.400 |
The patients who received rituximab as first line treatment were compared with those who received it after being refractory to DCP [Table/Fig-4]. There was a significant difference in the duration of disease between the two groups. There was no significant difference in gender, age, extent of disease early and late end points between the two groups. However, statistically significant number of refractory patients relapsed [Table/Fig-5].
Comparing disease parameters and end points between refractory and naïve patients after rituximab.
| Paired Samples | MEAN | N | S.D | S.Error Mean(S.E.M) | | Levene’s test for equality of variances | t test for equality of means | df | Sig(2 tailed) |
|---|
| F | Sig |
|---|
| Duration of disease (months) | Refractory | 18.90 | 10 | 9.871 | 3.121 | Equal variances assumed | 8.691 | 0.009 | 4.005 | 18 | 0.001 |
| Naïve | 4.50 | 10 | 5.642 | 1.784 |
| Time taken to attain disease control (weeks) | Refractory | 5.20 | 10 | 4.803 | 1.519 | 11.914 | 0.003 | 2.037 | 18 | 0.057 |
| Naïve | 2.10 | 10 | 0.316 | 0.100 |
| Duration of consolidation phase (months) | Refractory | 1.80 | 10 | 0.789 | 0.249 | 1.440 | 0.246 | 1.342 | 18 | 0.196 |
| Naïve | 1.40 | 10 | 0.516 | 0.163 |
| Duration of remission off treatment(weeks) | Refractory | 10.20 | 10 | 4.849 | 1.533 | 2.908 | 0.105 | -0.945 | 18 | 0.357 |
| Naïve | 12.70 | 10 | 0.816 | 2.155 |
Box chart comparing disease parameters and end points in naïve and refractory patients.
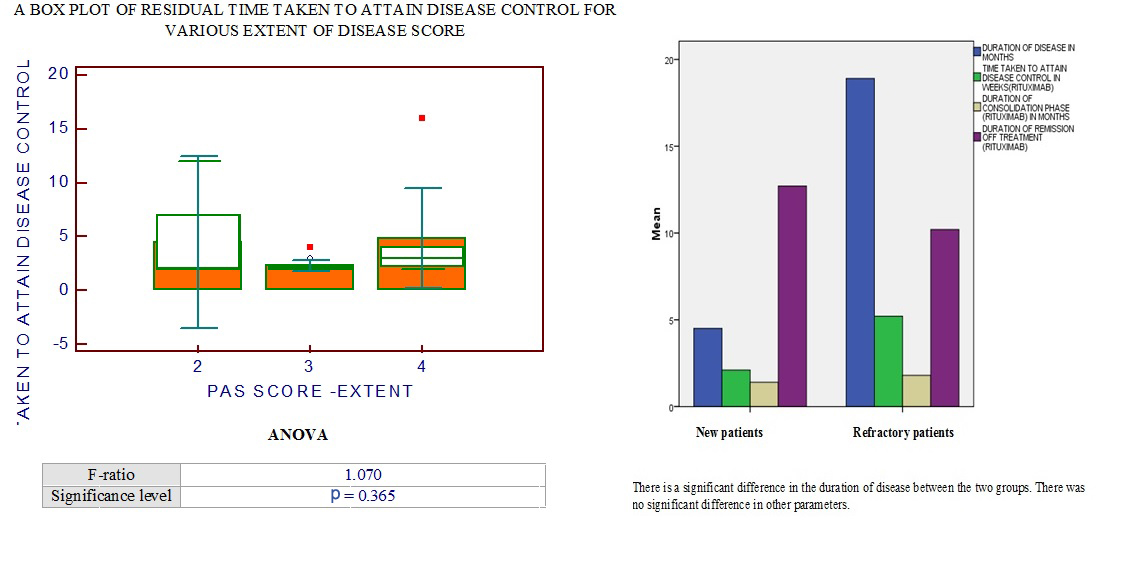
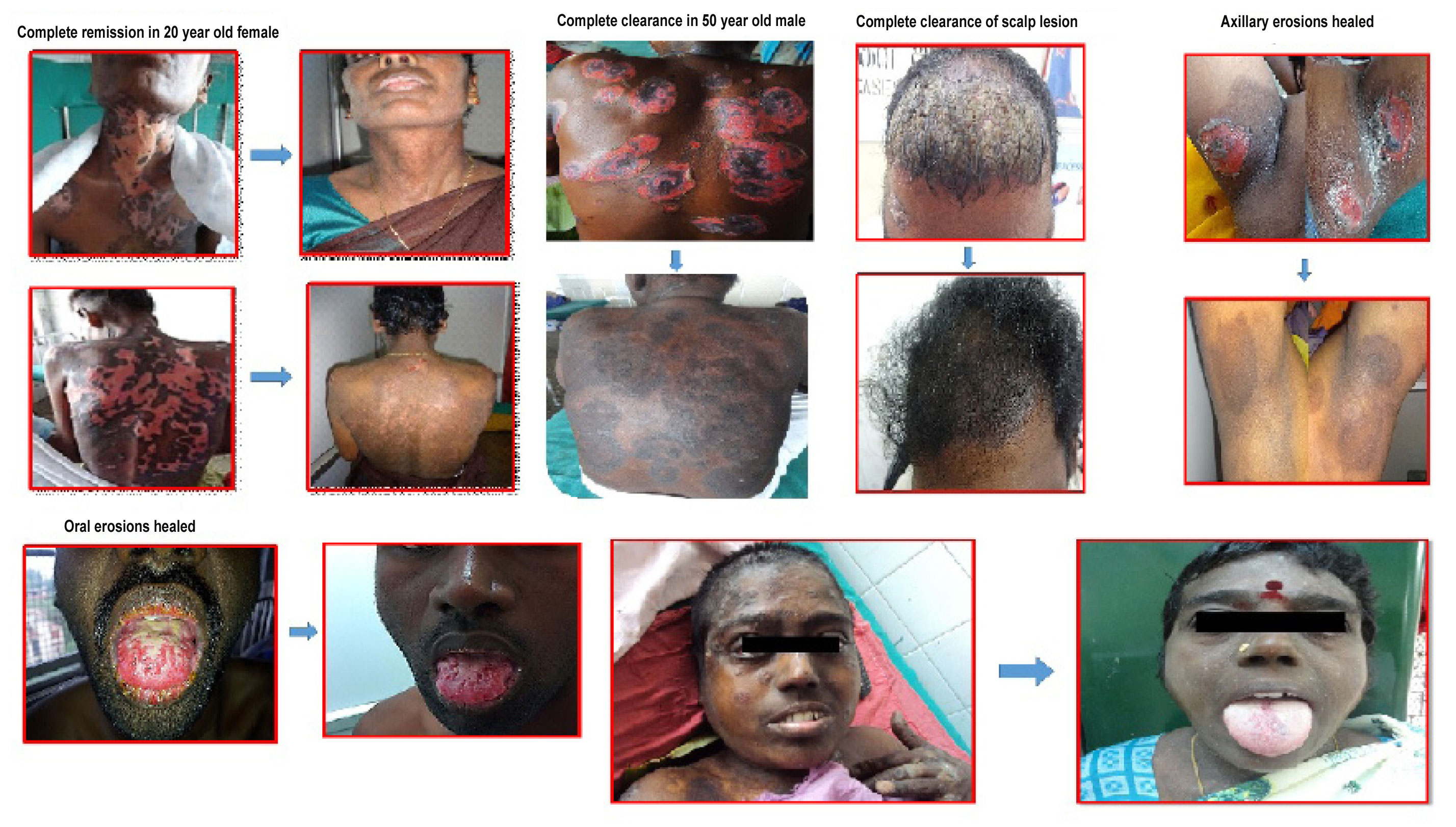
Out of 20 patients in the study, 13 were in complete remission till the end of the study period. Seven patients relapsed, of whom six had received rituximab after being refractory to conventional treatment for an average period of 11.7 months. One patient who received rituximab as first line therapy relapsed after 16 months. Patients who relapsed had a mean age of 43 years, mean disease duration of 21 months before rituximab and a mean PAS of 6.85. In the remission group, the mean age was 40 years, mean disease duration was 6.384 months, the mean PAS was 7.07. The end points of the two groups are tabulated [Table/Fig-6]. There was no significant difference in gender, age and extent of disease among patients who relapsed and those who attained remission. There was a significant difference in duration of disease, time taken to attain disease control and duration of consolidation phase between the two groups. However, the duration of remission off treatment was not significantly different [Table/Fig-7]. [Table/Fig-8] shows the pictures taken before and after rituximab.
Comparing disease parameters and end points in relapse and remission groups.
| Paired Samples | MEAN | N | S.D | S.Error Mean(S.E.M) | | Levene’s test for equality of variances | t test for equality of means | df | Sig(2 tailed) |
|---|
| F | Sig |
|---|
| Duration of disease (months) | Relapse | 21.57 | 7 | 11.631 | 4.396 | Equal variances assumed | 3.988 | 0.061 | 4.051 | 18 | 0.001 |
| Remission | 6.38 | 13 | 5.316 | 1.474 |
| Time taken to attain disease control (weeks) | Relapse | 6.29 | 7 | 5.469 | 2.067 | 30.527 | 0.000 | 2.722 | 18 | 0.014 |
| Remission | 2.23 | 13 | 0.439 | 0.122 |
| Duration of consolidation phase (months) | Relapse | 2.14 | 7 | 0.690 | 0.261 | 0.210 | 0.652 | 3.186 | 18 | 0.005 |
| Remission | 1.51 | 13 | 0.480 | 0.133 |
| Duration of remission off treatment(weeks) | Relapse | 10.00 | 7 | 3.786 | 1.431 | 4.822 | 0.041 | -0.799 | 18 | 0.435 |
| Remission | 12.23 | 13 | 6.784 | 1.882 |
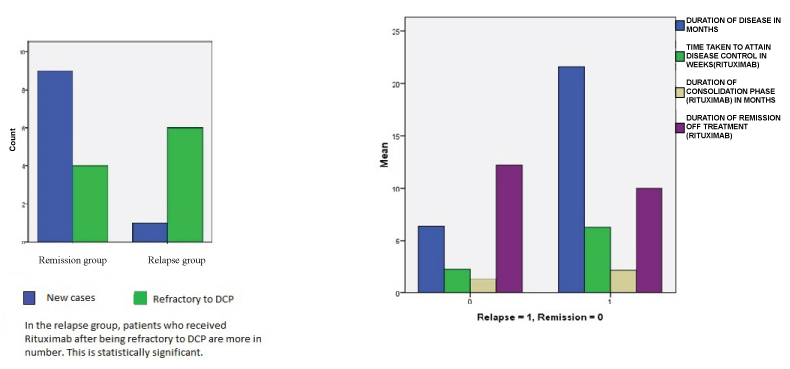
Box chart comparing disease parameters and end points in relapsed patients and in those who remained in remission.
Clinical pictures taken before and after rituximab.
No life threatening immediate adverse effects were noted in any patient. However, two patients (10%) developed bradycardia and hypotension during the infusion, which was successfully managed by decreasing the infusion rate. During follow up, one patient developed erythema nodosum, one patient developed pulmonary tuberculosis, four (20%) developed herpes labialis and two patients developed onychomycosis. Though, reactivation of herpes and pulmonary tuberculosis can occur following immunosuppression, we do not know if the erythema nodosum and onychomycosis occurred following rituximab infusion. As these were not noted in the patients when they received DCP therapy and occurred during the follow up period after rituximab infusion, we considered them to be possible adverse events [8]. All adverse reactions were managed appropriately.
Discussion
Pemphigus is a chronic autoimmune blistering disease with antibodies against desmoglein antigens produced by autoreactive B-cells. Rituximab binds to the CD20 cell surface receptor of B-cells and destroys them [3]. Rituximab is now being used as an adjuvant to pemphigus refractory to conventional treatment and also as a first line therapy. In our study, we have evaluated the effectiveness of rituximab in both refractory and naïve patients.
The mean age of the patients in our study was 41 years, slightly higher than that found in other Indian studies with an earlier age of onset. Most patients were less than 40 years in studies by Mascarenhas MF et al., and Singh R et al., only nine patients (45%) in our study were less than 40 years [9,10].
There was a female predominance in our study. Studies by Singh R and Sehgal VN have shown a male preponderance [10,11], Mascarenhas MF et al., and Kanwar AJ et al., has female predominance [9,12], while Kanwar A in another study found no sex preponderence in pemphigus [1].
In our study, it was found that the duration of disease before administering rituximab had a significant effect on the early and late end points and on relapse. This is in accordance with studies by Lunardon I et al., and Cho HH who concluded that rituximab is effective when given early in the course of disease [13,14]. As in those studies, in our patients also, the extent of the disease or the total PAS had no significant effect on the end points.
Rituximab has a significant effect in lowering the PAS from baseline to that at one and three months. The continued lowering of PAS after one month when steroids and immunosuppressants have been tapered off can be attributed to the effect of rituximab in prolonging remission. Zambruno G et al., attributed the long term remission seen after rituximab infusion to various mechanisms including destruction of autoreactive B and T cells [3], decrease in anti-desmoglein antibody level, delay in maturation of B cells and the naïve and immature repopulating B cells.
Rituximab shortens the consolidation phase and the need for additional steroids. Joly P et al., in his study showed that rituximab decreased the dose of oral prednisolone required from 94 to 12 mg per day in patients with refractory disease [15]. In another study, Craythorne et al., was able to reduce the dose of additional azathioprine [16].
It was found that patients who relapsed took a significantly longer time to attain disease control, went through a longer duration of consolidation phase and had a shorter duration of remission off- treatment whereas the remission group had short early end points and prolonged late end points [Table/Fig-6]. Colliou N et al., on comparing complete responders with incomplete responders after rituximab found that complete responders had profound depletion of antidesmoglein antibodies and auto reactive B cells and more of immature and naïve B cells which contributed to the early and sustained remission [17].
There was no effect of age, sex, disease extent or PAS on the end points of the study and there were no differences in these parameters between the people who relapsed and those who remained in remission. This was similar to the above studies.
Compared with the ten DCP refractory patients receiving rituximab, the ten new cases receiving it as firstline therapy had significantly less number of relapses and more remained in remission. A study by Cho YT et al., also shows that rituximab given as first line therapy along with steroids is very effective [18]. The early and late end points for conventional treatment and rituximab were compared in those patients who received both. Rituximab was more effective than conventional treatment in attaining an early and sustained remission.
Leshem YA et al., showed that a single cycle of rituximab as per the RA protocol produced a complete remission rate of 76% and a relapse rate of 22% at a mean time of eight months [19]. The remission rate was increased to 91% after the second cycle of rituximab. In our study, a single cycle of rituximab as per RA protocol produced a complete remission of 65% and relapse rate of 35%. Three patients received additional cycles of rituximab when they relapsed. One patient relapsed nine months after the second cycle and was given a third cycle. A second cycle of rituximab increased the remission rate to 80%. However, the study group of Leshem YA et al., had new cases of pemphigus who were treated with rituximab in addition to steroids and other immunosuppressants, whereas our study group had both new cases and patients refractory to conventional treatment. This may contribute to the lower remission and higher relapse rate observed in our study.
Londhe PJ et al., has used rituximab according to lymphoma protocol in pemphigus patients with refractory disease in her study [20]; 50% showed complete remission off therapy, 42% partial remission and 8% relapsed at the end of study period. In our study, the complete remission rate was slightly higher (65%). The relapse rate was also higher at 35%. None were in partial remission at the end of the study.
The overall incidence of adverse effects was 1-16% in various studies [13,14,21–25]. In our study, 35% developed a significant adverse effect. As about 20% of the patients developed a reactivation of herpes simplex virus, prophylactic acyclovir suppression therapy can be given after rituximab infusion. Prophylactic cotrimoxazole can also be given for three to six months in view of increased susceptibility to bacterial infections. There was no life threatening adverse reaction.
Limitation
The limitation of our study was the small sample size and variable duration of follow up. Most of the patients who relapsed did so at six to 12 months after rituximab infusion. Hence, further studies may be required based on serological investigations, to delineate those patients who require additional doses of rituximab at regular intervals for sustained remission.
Conclusion
Rituximab given in pemphigus as an adjuvant to corticosteroids, was effective in significantly decreasing the disease activity (as measured by PAS before and after treatment) in both refractive and new cases. It decreased the need for additional steroids and other immunosuppressants and prolonged remission. The duration of disease before rituximab infusion had a significant effect on its effectiveness, being effective when given early in the disease process. Patients who take longer to attain disease control and complete consolidation phase, relapse early. Rituximab was significantly better than conventional treatment in refractory patients. Rheumatoid arthritis protocol was effective in producing sustained remission for more than one year in nearly 50% of the patients with no serious adverse effects. Thus, rituximab proves to be a magic bullet for pemphigus. Further studies are needed to know the need for and dose of additional cycles of rituximab to maintain prolonged remission.
*- Pemphigus Activity Score.
*- Standard Deviation; * - Standard Error Mean; $ - Degree of freedom