Anaplastic Pleomorphic Xanthoastrocytoma in a Case of Neurofibromatosis Type 1: A Case Report
K Thara1, Reetika Sharma2, G Thiagarajan3, Anita Ramdas4, Renu Gboy Varghese5
1 Postgraduate, Department of Pathology, Pondicherry Institute of Medical Sciences, Puducherry, India.
2 Assistant Professor, Department of Pathology, Pondicherry Institute of Medical Sciences, Puducherry, India.
3 Professor, Department of Neurosurgery, Pondicherry Institute of Medical Sciences, Puducherry, India.
4 Professor, Department of Pathology, Pondicherry Institute of Medical Sciences, Puducherry, India.
5 Professor, Department of Pathology, Pondicherry Institute of Medical Sciences, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. K Thara, Room no: 534, OPD block, Pondicherry Institute of Medical Sciences, Puducherry-605014, India.
E-mail: thara.keloth@gmail.com
Pleomorphic Xanthoastrocytoma (PXA) is a rare brain tumour comprising only <1% of primary brain tumours which is seen in children and young adults. Only 9-20% of the PXA shows anaplastic features and this has a bad prognosis. PXA is a WHO grade II tumour while anaplastic PXA is a WHO grade III tumour. Neurofibromatosis type 1(NF1), which is an autosomal dominant condition, predisposes to tumours of the central nervous system; most of which are pilocytic astrocytomas. Association of PXA with NF1 is very rare and only a very few cases have been reported. Here, we present a case of 42-year-old male, a known case of NF1, with multiple neurofibromas, who presented with right sided hemiparesis, seizures and vomiting. The histopathology and immunohistochemistry features were suggestive of anaplastic PXA.
Central nervous system tumour, High grade, Glioblastoma, Immunohistochemistry
Case Report
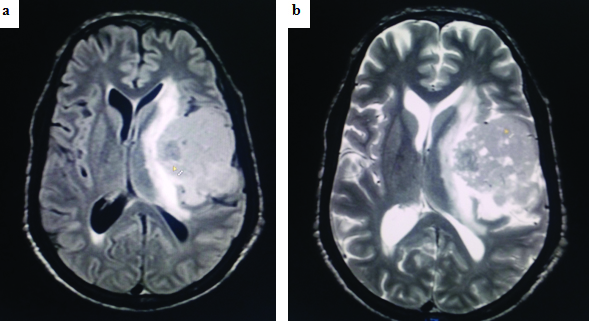
A 42-year-old male, a known case of Neurofibromatosis type 1 (NF1), presented with progressive weakness of the right side of the body over a period of six months. He had complaints of giddiness and headache for three months. He also had few episodes of seizures and vomiting for 15 days. Family history revealed presence of neurofibromatosis in his father and sister. His general physical examination showed multiple neurofibromas all over the body. Ophthalmic examination was normal. Neurological examination showed right sided weakness. Computed Tomography (CT) of brain showed a large ill-defined iso-intense mass in the left tempero-parietal region measuring 8 x 5.3 x 4.5 cm with mild midline shift. Magnetic Resonance Imaging (MRI) of brain [Table/Fig-1] with contrast was subsequently done which revealed a well-defined lesion in the left tempero-parietal lobe, with a predominantly solid component and with tiny cystic areas which showed moderate enhancement on contrast. Mild perilesional oedema with midline shift was present. Another small, mildly hyper-intense lesion of 1.6 x 1.3 cm which showed no contrast enhancement was present in the right cerebellum. Left frontal craniotomy and excision of the tempero-parietal mass was done. The right cerebellar mass was not excised. There were no complications in the postoperative period. We lost follow up of the patient.
(a) MRI image (FLAIR) and (b) MRI image (T2) showing iso-intense lesion in the left tempero-parietal region with predominant solid component with tiny cystic areas with mild contrast enhancement in MRI with peri-lesional oedema and mid-line shift.
Pathological Findings
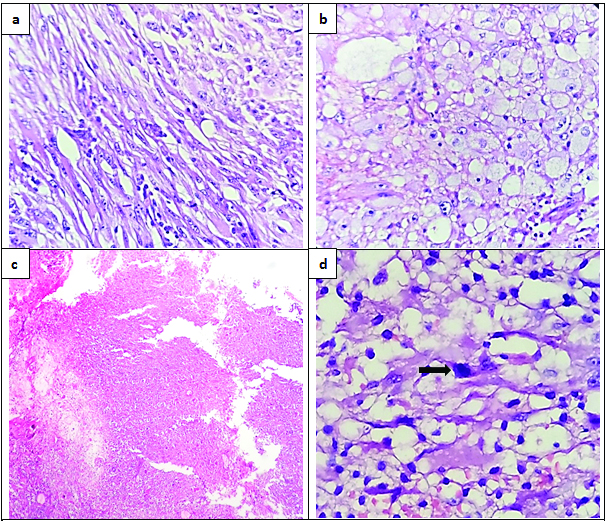
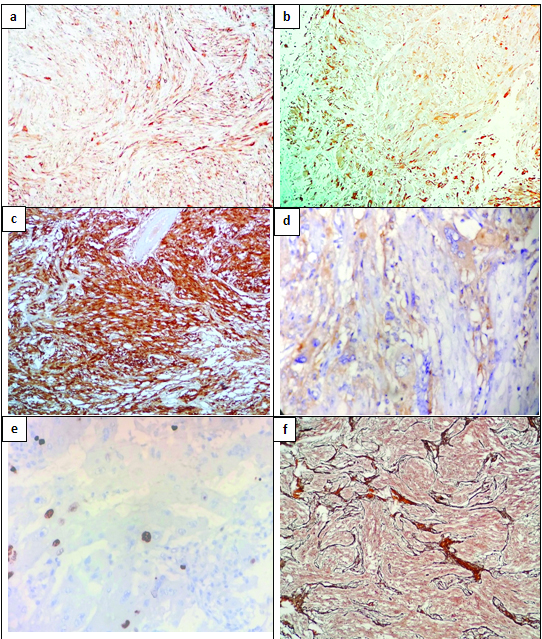
The histopathological examination showed tumour fragments composed of spindle cells having elongated nuclei arranged in interlacing fascicles and polygonal cells with abundant vacuolated cytoplasm [Table/Fig-2a,b]. There was marked pleomorphism with presence of bizarre cells and multinucleated giant cells. Necrosis was present, but there was no pseudopalisading of tumour cells [Table/Fig-2c]. Mitosis was 7/10 hpf [Table/Fig-2d]. Tumour stroma had lymphocytic infiltration. Glial Fibrillary Acidic Protein (GFAP) showed positivity in spindle cell component and xanthomatous cells [Table/Fig-3a,b]. S-100 also showed diffuse positivity [Table/Fig-3c]. Synaptophysin showed focal positivity [Table/Fig-3d]. MIB-I index showed a proliferation index of 7-10% [Table/Fig-3e]. Reticulin showed diffuse positivity [Table/Fig-3f]. Based on the histological features and immunohistochemistry findings, a diagnosis of anaplastic pleomorphic xanthoastrocytoma was made.
Histological sections showing: (a) Spindle cell component (H&E stain 10X); (b) Cells with vacuolated cytoplasm (H&E stain 10X); (c) Geographic necrosis with no pseudo pallisading of cells (H&E stain 4X); (d) A mitotic figure (arrow) (H&E stain 40X).
(a) Spindle cell component showing GFAP positivity (IHC 10X); (b) Vacuolated cells showing GFAP positivity (IHC 10X); (c) Tumour cells showing diffuse S-100 positivity (IHC 10X); (d) Synaptophysin showed focal positivity (IHC 10X); (e) Ki-67 showed proliferative index of 7-10% (IHC 40X); (f) Reticulin stain with diffuse positivity (Gordon and Sweet stain, 10X).
Discussion
PXA is a rare Central Nervous System (CNS) neoplasm that accounts for 1% of all astrocytic tumours [1,2]. The term PXA was coined in 1979 by Kepes and co-authors [1,3]. It was added in the 1993 World Health Organization (WHO) classification of tumours of the CNS and was first described in 1973 [1]. PXA belongs to grade II of the WHO classification of tumours of the CNS with a favourable prognosis. It usually affects children and young adults and is usually supratentorial and superficially located [1,4].
Radiologically, most of the PXAs (70%) appear as a cyst with solid mural nodule with enhancement on Gadolinium (Gd) administration, but our case showed predominantly solid areas with tiny cystic areas, which is documented in 30% of the cases [1].
Even though it is a low grade astrocytic tumour, histologically it shows marked cellular and nuclear pleomorphism. Histological features of PXAs are pleomorphic cells with vacuolated cytoplasm (xanthomatous cells), spindle cells arranged in fascicles, eosinophilic granular bodies, bizarre looking cells and multinucleate giant cells with reticulin rich stroma and lymphocytic cuffing [1-3,5]. PXA with five or more mitoses per 10 high power fields was termed as PXA with Anaplastic Features (PXA-AF) previously, but it is added in the 2016 WHO classification of CNS tumours as anaplastic PXA and is given a higher grade (Grade III). Necrosis may be present, but the significance of necrosis in the absence of mitoses is uncertain [6].
Immunohistochemically, PXA shows positivity for both neuronal and glial markers. CD34 positivity may be present. EGFR and p53 are not expressed in PXA, while these show positivity in glioblastoma. MIB-I shows a low proliferative index, usually less than 5% [1,5,7]. In our case the MIB-I index was about 7-10%, which is consistent with anaplastic PXA.
The important differentials for anaplastic PXA are, Malignant Fibrous Histiocytoma (MFH) and glioblastoma [2,4,7]. The GFAP and S-100 positivity in our case rules out MFH. Even though our case had necrosis, it lacked geographical necrosis with pseudopalisading of tumour cells and endothelial hyperplasia, which are the characteristic features of glioblastoma. Synaptophysin positivity in our case also helped to rule out glioblastoma.
NF1 is an autosomal dominant genetic disorder involving the chromosomal 17q11.2 gene locus. The prevalence of NF1 in the world is between 1/2000 to 1/8000 populations. The features include café-au-lait spots, subcutaneous neurofibromas (usually multiple), Lisch nodules of the iris, and bone lesions. NF1 predisposes to development of intracranial tumour, mainly pilocytic astrocytoma [1]. The first case of PXA in association with NF1 was reported in 1993 in a 14-year-old boy from Turkey [1]. Our case is a PXA in association with NF1 which is rarely present.
Conclusion
The incidence of PXA in NF1 is very rare with only a few cases reported till date. After thoroughly reviewing the literature, to the best of our knowledge only ten cases of PXA have been reported in association with NF-1. We hereby report another case of anaplastic PXA in association with NF-1 with atypical radiological features.
[1]. Phalke U, Gotecha S, Sagale M, Ganjare P, Punia P, Sharma S, Pleomorphic xanthoastrocytoma in a patient with Neurofibromatosis type 1International Journal of Advances in Case Reports 2015 2:617-20. [Google Scholar]
[2]. Niamathullah S, Sivaselvam S, Ghosh M, Ghosh S, Pleomorphic xanthoastrocytoma with anaplastic features: a case reportIndian J Pathol Microbiol 2014 57:101-04. [Google Scholar]
[3]. Martinez-Diaz H, Kleinschmidt-DeMasters BK, Powell ZS, Yachnis AT, Giant cell glioblastoma and pleomorphic xanthoastrocytoma show different immunohistochemical profiles for neuronal antigens and p53 but share reactivity for classIII beta-tubulinArch Pathol lab Med 2003 127:1187-91. [Google Scholar]
[4]. Takei H, Rouah E, Bhattacharjee MB, Cerebellar pleomorphic xanthoastrocytoma in a patient with Neurofibromatosis type 1: a case report and literature reviewInt J Clin Exp Pathol 2015 8:7570-74. [Google Scholar]
[5]. Saikali S, Le Strat A, Heckly A, Stock N, Scarabin JM, Hamlat A, Multicentric pleomorphic xanthoastrocytoma in a patient with Neurofibromatosis type 1. Case report and review of the literatureJ Neurosurg 2005 102:376-81. [Google Scholar]
[6]. Louis DN, Perry A, Reifenberger G, von Deiming A, Figarella-Branger D, Cavenee WK, The 2016 World Health Organization Classification of Tumours of the Central Nervous System: a summaryActa Neuropathol 2016 131:803-20. [Google Scholar]
[7]. Kumar S, Retnam TM, Menon G, Nair S, Bhattacharya RN, Radhakrishnan VV, Cerebellar hemisphere, an uncommon location for pleomorphic xanthoastrocytoma and lipidized glioblastoma multiformisNeurol India 2003 51:246-47. [Google Scholar]