Cerebral Vein Thrombosis (CVT) is an uncommon cause of stroke. Thrombosis can occur in superficial veins, deep venous system or cortical veins of brain. The term Deep Cerebral Vein Thrombosis (DCVT) is used for thrombosis of internal cerebral vein, vein of Galen and basal vein of Rosenthal. Only 10% cases of CVT are because of thrombosis of deep cerebral vein. The diagnosis of DCVT is often missed because of its heterogenous presentation. Herein, we present a case of DCVT which was initially treated as meningoencephalitis. A timely advised brain imaging helped in making the diagnosis and patient recovered completely after institution of anticoagulation.
Hyperhomocystinemia, Meningoencephalitis, Warfarin
Case Report
A 54-year-old male presented to the Medical Emergency Department with complaints of generalized headache and projectile vomiting for two days which was followed by altered sensorium. He also gave history of low grade fever of one day duration. He was given various over the counter medication for these complaints, but his symptoms didn’t abate. There was no history of seizure, trauma, rash, recent travel or tuberculosis in past. He was a non vegetarian by diet and an occasional drinker.
On examination, his pulse rate was 80 /minute, regular and blood pressure was 110/70 mmHg in right arm supine position. He was afebrile and had Glasgow Coma Scale (GCS) of E2V1M3. Central Nervous System (CNS) examination showed extensor plantar response on right side and terminal neck rigidity. However, there was no motor deficit or cranial nerve palsy and fundus examination was normal. Rest of the systemic examination was essentially normal. A possibility of CNS pathology was kept and further investigations were carried out. Non Contrast Computed Tomography (NCCT) of head was done, which was normal and other routine blood investigations like hemogram, renal function tests, liver function tests, serum electrolytes were also normal. A lumbar puncture was done and CSF analysis showed sugar of 71 mg/dl, protein - 214 mg/dl with 20 cells/cumm (mononuclear cells 80%, polymorphs 20%). Based on these primary investigations, we narrowed down our differential to viral meningoencephalitis and treatment was started immediately for the same. He was given injection acyclovir at dose of 10 mg/kg q8 hourly, along with injection dexamethasone, ceftriaxone and mannitol. However, his sensorium further deteriorated in next 48 hours and other tests of Cerebrospinal fluid (CSF) like; Adenosine Deaminase (ADA) and Genexpert for tuberculosis, Japanese Encephalitis (JE), Herpes Simplex Virus (HSV) also came negative.
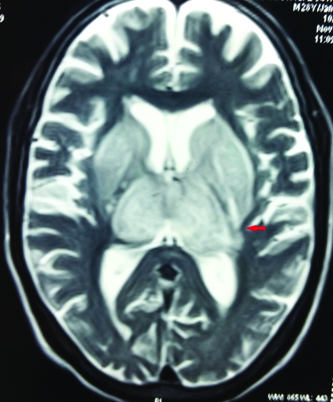
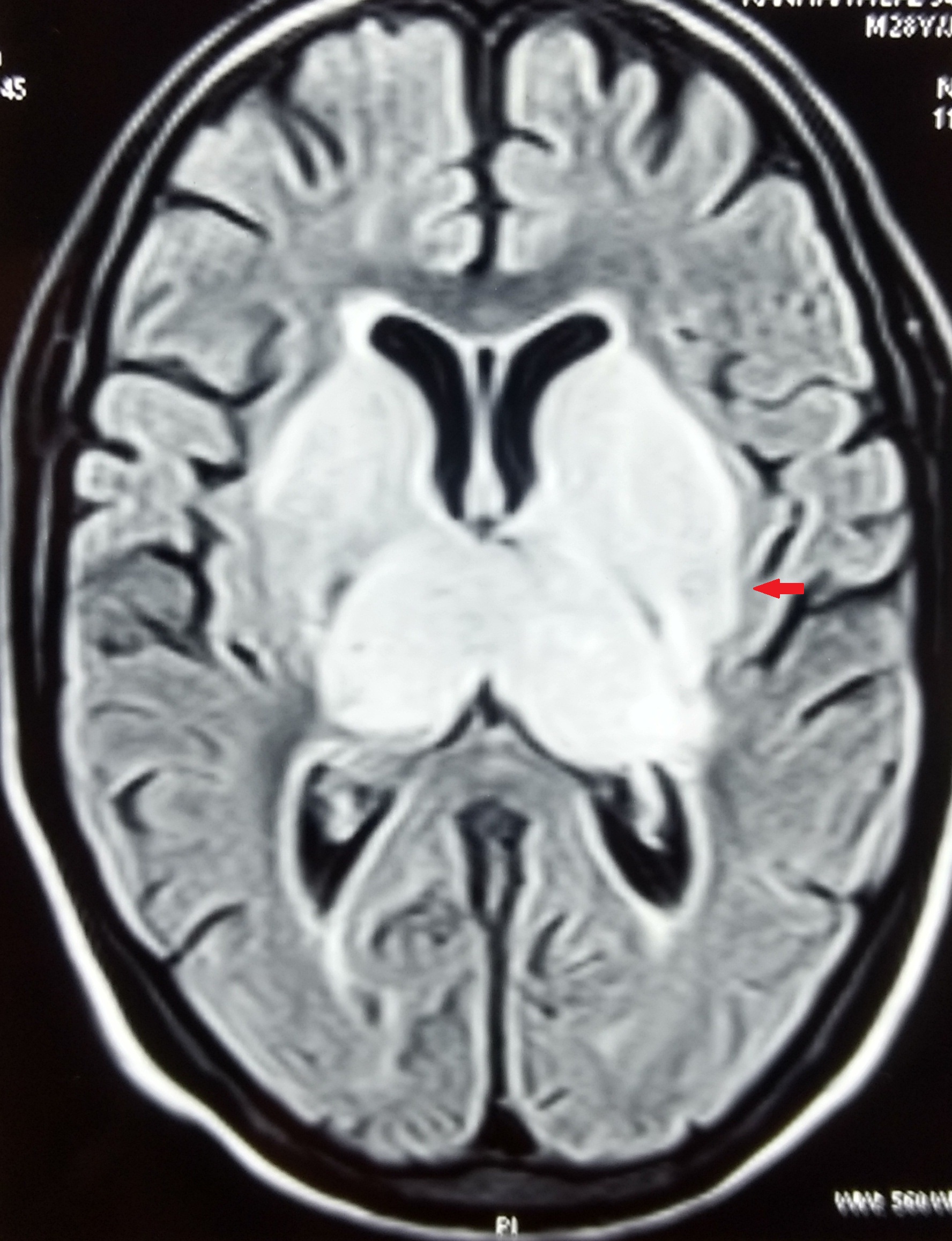
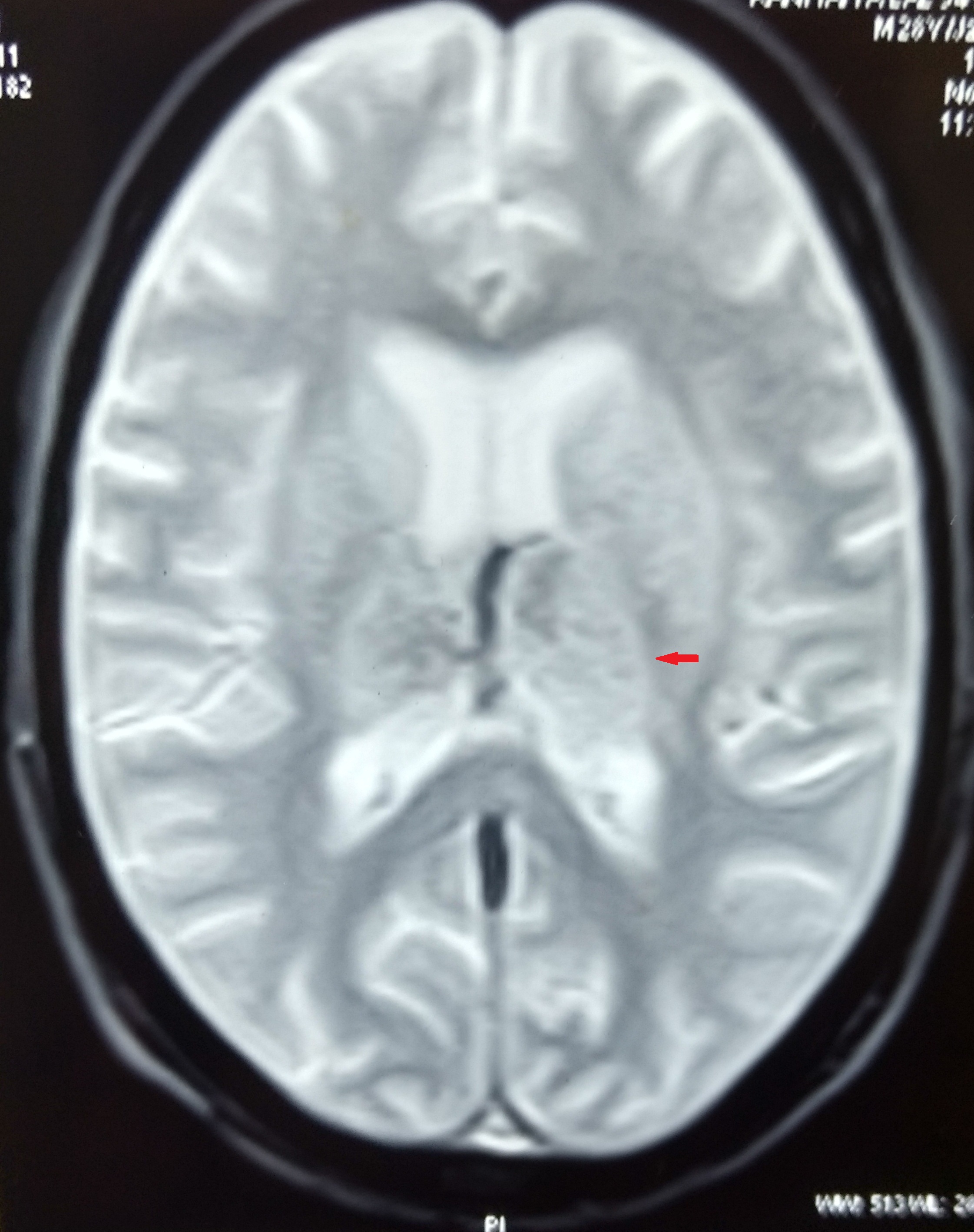
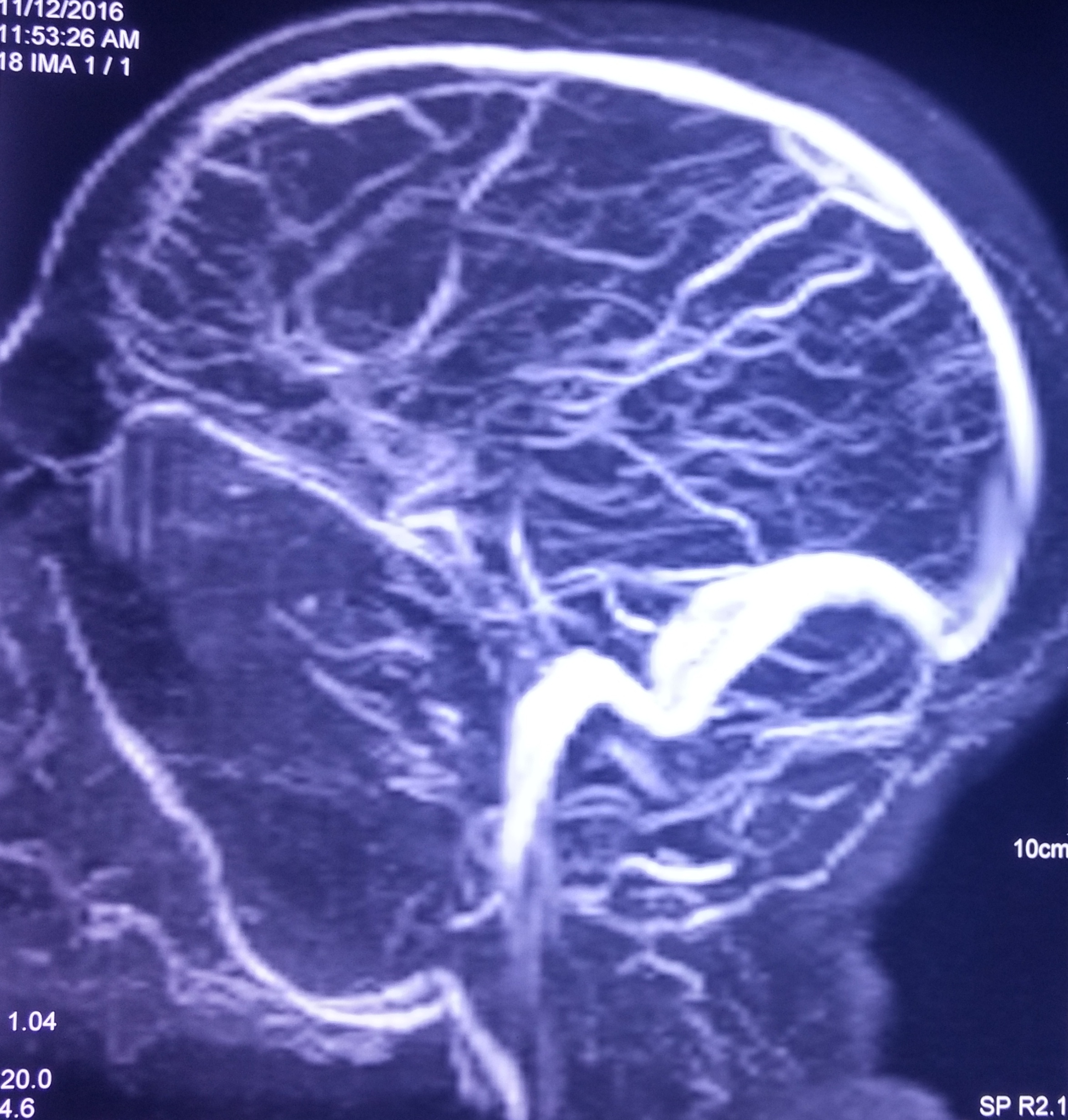
At this juncture, we decided to get Contrast Enhanced MRI (CEMRI) of brain, which showed symmetrical altered signal intensity in the form of T2/flair hyperintensity and T1 hypointensity involving the bilateral thalami and basal ganglia and patchy diffusion restriction on diffusion weighted images with patchy foci of blooming on Gradient Recalled Echo (GRE) images, likely hemorrhagic foci secondary to thrombosis [Table/Fig-1,2,3 and 4]. Bilateral internal cerebral veins and basal vein of Rosenthal showed hypointensity on T2W images and isointensity on T1W images. MRI findings were grossly pointing towards DCVT. Subsequently, an MR Venography (MRV) was done which clinched the diagnosis. MRV showed thrombosis of straight sinus, bilateral internal cerebral vein and vein of Galen [Table/Fig-5].
T1W image showing hypointensity in bilateral thalami and basal ganglia.
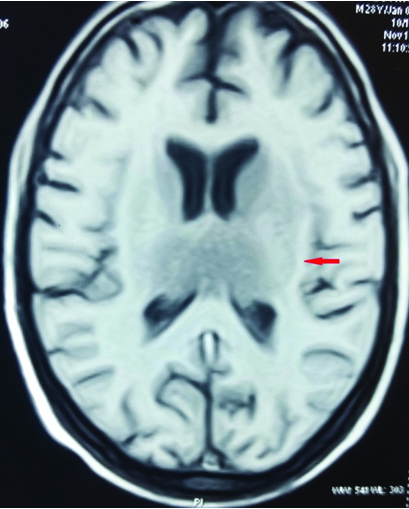
T2W image showing hyperintensity in bilateral thalami and basal ganglia.
Bilateral thalami and basal ganglia hyperintensity on FLAIR images.
Blooming seen on GRE images.
Non visualization of deep cerebral veins on MRV.
He was immediately started on bridging therapy of anticoagulation with Low Molecular Weight Heparin (LMWH) and oral warfarin. He showed a momentous improvement in sensorium and had GCS of 15 by seventh day of anticoagulation. He was discharged on oral warfarin (2 mg once daily) and was advised to maintain International Normalised Ratio (INR) between two to three. After six weeks, tests for thrombophilic states were done in which serum homocysteine level was markedly elevated and other factors were normal. Vitamin B12, pyridoxine and folate supplementation were added in his treatment regime for hyperhomocystinemia.
Discussion
CVT is an uncommon cause of stroke which affects almost 3-4 adults per million population annually [1]. Although, there are no population based studies from India estimating incidence of CVT, various hospital based studies have found that CVT comprises 10% of all stroke cases in our country [2]. Superior sagittal sinus is the most commonly affected sinus followed by transverse and sigmoid sinus. DCVT term is used for thrombosis of internal cerebral vein, vein of Galen, basal vein of Rosenthal and its tributaries, and is seen in 10% cases of CVT [3]. There are various predisposing factors that combine together and results in thrombus formation in cerebral veins. Inherited thrombophilic states, hyperhomocystinemia, dehydration, drugs, pregnancy, malignancy and autoimmune conditions are often found in CVT patients. However, in 15% cases of CVT, no direct cause or predisposing factor could be identified [4].
Due to varied clinical presentation, diagnosis of CVT is often missed or delayed. Headache is the most common symptom and is seen in almost 70%-90% cases [5]. Hemiparesis, seizures, loss of consciousness, papilloedema and visual disturbances are other commonly observed presentation in CVT. The clinical presentation of DCVT is non specific and patient can present with short history of deteriorating consciousness, aphasia, seizures, nausea, vomiting, coma or death [6]. It is often difficult to differentiate DCVT, clinically from other common causes of altered sensorium like metabolic encephalopathy or meningoencephalitis.
The parenchymal changes in CVT result from increased venous pressure which leads to vasogenic oedema, cytotoxic oedema and hemorrhagic infarction. CT finding in DCVT often shows hypoattenuation of bilateral thalami and basal ganglia. However, the venous system which includes vein of Galen, vein of Rosenthal, internal cerebral vein and straight sinus appear hyperattenuated on CT [7]. The most sensitive imaging technique for early diagnosis of DCVT is MRI with MRV [8]. The deep venous system drains thalamus, basal ganglia, corpus callosum, inferior frontal lobes and deep white matter of parietal and temporal lobe [9]. So, thrombosis of deep cerebral vein causes vasogenic oedema and ischemic changes in these area, which appears hyperintense on T2-weighted images and hypointense on T1-weighted images. Based on signal intensity on T1 and T2 weighted images, venous thrombosis can be divided in initial, intermediate and late stage. In first five days (initial stage), there is absence of flow-void and vessel appear isointense on T1 and hypointense on T2 weighted images. Beyond five days (intermediate stage), there is absence of flow with hyperintensity on both T1 and T2 weighted images. After two weeks (late stage), recanalization occurs with resumption of flow-void in abnormal vessels [10].
MRI involvement of bilateral thalamus is seen in various other conditions like JE, Wilson’s disease, Wernicke’s encephalopathy, hypoxic-ischemic encephalopathy and autoimmune demyelinating conditions like multiple sclerosis [11]. MRI finding of JE closely resembles that of DCVT and shows hyperintense lesion in bilateral thalami on T2-weighted images, while hemorrhagic transformation appears hyperintense on T1-weighted image. The oedema is predominantly cytotoxic as compared to vasogenic oedema in DCVT. MRI imaging often shows thrombus in deep cerebral vein in case of DCVT which can be confirmed further by MRV. MRV shows abnormal signal intensity in corresponding sinus with absence of flow [8]. CSF analysis in CVT is often abnormal and shows increased protein level with lymphocytic pleocytosis, which almost mimics CSF picture of viral meningoencephalitis [3]. So, initial clinical picture and CSF analysis of DCVT can be easily confused with that of meningoencephalitis. However, negative test for viral antigen in CSF and MRI imaging helps in identifying DCVT. Any delay in diagnosis of DCVT can be lethal and mortality rate increases several folds if anticoagulation is not started in initial days. Untreated patient often develops features of raised intracranial pressure and brain herniation which can lead to permanent residual disability and often death.
Acute thrombosis decreases level of antithrombin, protein C and protein S, so test for thrombophilic states should be performed at least six weeks after an acute thrombotic event [12]. Treatment of DCVT remains anticoagulation with heparin/LMWH initially, followed by warfarin to maintain an INR between two to three. Mannitol and dexamethasone can be used as antioedema measure. Any underlying correctable cause of hypercoagulability should be treated accordingly. For provoked CVT, duration of vitamin K antagonist is three to six months. Indefinite therapy is recommended in presence of severe thrombophilia (protein C or S deficiency, antithrombin deficiency, homozygous factor V leiden mutation, antiphospholipid syndrome), recurrent CVT and venous thromboembolism after CVT. For remaining cases of CVT, six to twelve month anticoagulation is recommended.
In our case, the clinical presentation and CSF analysis was suggestive of viral meningoencephalitis but a diagnosis of DCVT was made after MRI and MRV of brain. The possible cause of thrombosis was hyperhomocystinemia and dehydration.
Conclusion
A high index of suspicion is needed for early diagnosis of DCVT. It has favourable outcome, if recognized and treated early. MRI brain should be done early in cases of unexplained altered sensorium. If MRI is suggestive of DCVT, then MRV should be done for confirmation. Any reversible cause of thrombosis should be treated.
[1]. Stam J, Thrombosis of the cerebral veins and sinusesN Engl J Med 2005 352(17):1791-98. [Google Scholar]
[2]. Banerjee AK, Varma M, Vasista RK, Chopra JS, Cerebrovascular disease in north-west India: a study of necropsy materialJ Neurol Neurosurg Psychiatry 1989 52:512-15. [Google Scholar]
[3]. Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, ISCVT Investigators: prognosis of cerebral vein and dural sinus thrombosis: results of the international study on cerebral vein and dural sinus thrombosis (ISCVT)Stroke 2004 35:664-70. [Google Scholar]
[4]. Schaller B, Graf R, Cerebral venous infarction: the pathophysiological conceptCerebrovasc Dis 2004 18(3):179-88. [Google Scholar]
[5]. Ameri A, Bousser MG, Cerebral venous thrombosisNeurol Clin 1992 10(1):87-111. [Google Scholar]
[6]. Crawford SC, Digre KB, Palmer CA, Bell DA, Osborn AG, Thrombosis of deep venous drainage of the brain in adults: analysis of seven cases with review of the literatureArch Neurol 1995 52:1101-08. [Google Scholar]
[7]. Eick JJ, Miller KD, Bell KA, Tutton RH, Computed tomography of deep cerebral venous thrombosis in childrenRadiology 1981 140:399-402. [Google Scholar]
[8]. Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE, Cerebral MR venography: normal anatomy and potential diagnostic pitfallsAm J Neuroradiol 2000 21(1):74-78. [Google Scholar]
[9]. Meder JF, Chiras J, Roland J, Guinet P, Bracard S, Bargy F, Venous territories of the brainJ Neuroradiol 1994 21(2):118-33. [Google Scholar]
[10]. Macchi PJ, Grossman RI, Gomori JM, Goldberg HI, Zimmerman RA, Bilaniuk LT, High field MR imaging of cerebral venous thrombosisJ Comput Assist Tomogr 1986 10(1):10-15. [Google Scholar]
[11]. Kalita J, Misra UK, Comparison of CT and MRI findings in the diagnosis of Japanese encephalitisJ Neurol Sci 2000 174(1):3-8. [Google Scholar]
[12]. Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke AssociationStroke 2011 42(4):1158-92. [Google Scholar]