A Rare Presentation of Metastasis to the Thyroid Gland
Mekala Lakshminarayanan1, Ann Kurian2
1 Registrar, Department of Pathology, Apollo Speciality Cancer Hospital, Teynampet, Chennai, India.
2 Senior Consultant, Department of Pathology, Apollo Speciality Cancer Hospital, Teynampet, Chennai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mekala Lakshminarayanan, G1,“Surabhishri”, 5/9, Thiru Vi Ka Third Street, Mylapore, Chennai-600004, India.
E-mail: drlmekala@yahoo.co.in
The thyroid gland has a rich vascular supply. However, metastasis to the thyroid is exceedingly rare. The most common tumours that metastasize to the thyroid are breast and lung. The incidence of metastasis is on the rise due to better imaging techniques as well as procedures such as Fine Needle Aspitation Cytology (FNAC) and Immunohistochemistry (IHC). Secondaries from a prostate cancer are most commonly seen in bone, lung and liver. To our knowledge, metastasis from the prostate gland to the thyroid is exceedingly rare. Here, we report a case of metastatic prostatic adenocarcinoma to the thyroid gland two years after prostatectomy.
Fine needle aspitation cytology, Immunohistochemistry, Prostatectomy, Secondaries
Case Report
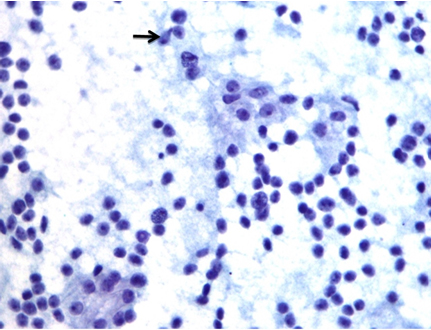
A 64-year-old gentleman, known case of adenocarcinoma of prostate, Gleasons’s combined score 7(4+3), who underwent robotic prostatectomy two years ago, presented to the Surgical Oncology Department for follow up. He had no specific complaints. Clinical examinations as well as routine investigations were within normal limits. His serum Prostate Specific Antigen (PSA) was 3.1ng/ml. Postoperatively his serum PSA dropped to undetectable levels and had remained so till this checkup. As the clinician suspected a local recurrence of the tumour, a Positron Emission tomography (PET) scan was performed which showed a small hypodense lesion of size 7-8 mm in inferior pole of left lobe of thyroid gland. Ultrasound guided FNAC from that site revealed cellular smears with singly dispersed and loose clusters of moderate to highly pleomorphic cells suggestive of anaplastic carcinoma [Table/Fig-1]. Thyroid function tests were within normal limits. Total thyroidectomy was done.
Cytology smear shows discohesive clusters and singly scattered atypical cells with high nucleocytoplasmic ratio, vesicular nuclei and prominent nucleoli (arrow).(Pap, 40X).
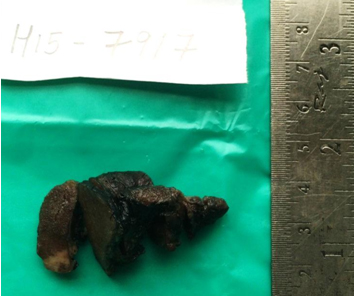
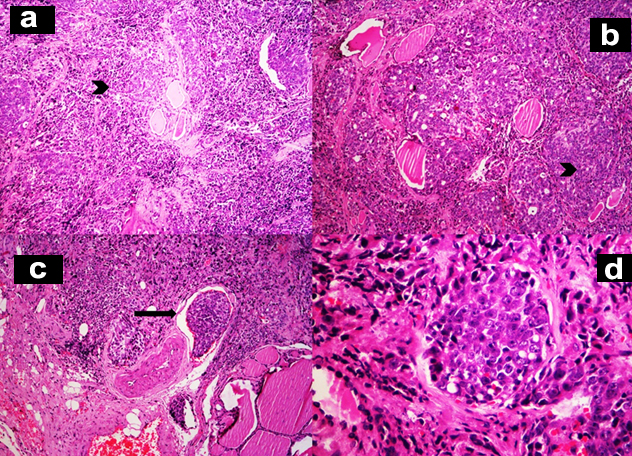
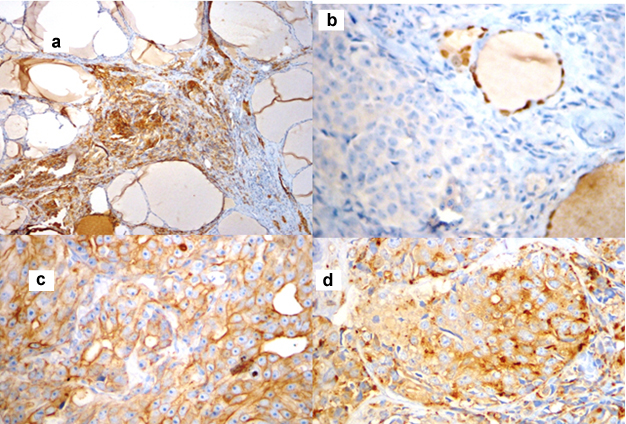
Grossly, the thyroid gland was mildly enlarged with right lobe measuring 6.5x3.5x1.5 cm and left lobe measuring 5.5x2x1.5 cm. Serial sections through the left lobe revealed a single, ill defined, grey white, firm lesion measuring 1.3x1x0.9 cm [Table/Fig-2]. Histopathological examination of this lesion revealed thyroid parenchyma with interstitial infiltrates of a malignant neoplasm composed of round to oval cells exhibiting high nucleocytoplasmic ratio, vesicular nuclei and prominent 1-2 nucleoli arranged in sheets, nests, vague glandular pattern, cords and singly. Multiple lymphatic emboli were noted [Table/Fig-3]. Immunohistochemistry (IHC) was performed. These atypical cells were positive for cytokeratin (CK), PSA and Prostatic Specific Acid Phosphatase (PSAP). Thyroid Transcription factor-1 (TTF-1) was negative [Table/Fig-4]. The final diagnosis was metastatic prostatic adenocarcinoma to the left lobe of thyroid gland.
Cut surface of thyroid gland showing ill defined grey white area in the inferior pole of left lobe.
a,b-Interstitium of thyroid gland is infiltrated by sheets and nests of atypical cells (H&E,x100); c-Lymphatic emboli are evident (H&E,x100); d-These atypical cells have high nucleocytoplasmic ratio, vesicular nuclei and prominent nucleoli (H&E,x400);
IHC patterns. a) Cells are positive for CK (10X); b) Tumour cells are negative for TTF-1.(10X); c) Cells are positive for PSA (40X); d) Cells are positive for PSAP (40X).
Post total thyroidectomy, the serum PSA reduced to 0.2 ng/ml. There were no postoperative complications. After surgery, the patient has completed one cycle of chemotherapy and is doing well till date.
Discussion
Inspite of having a rich vascular supply, thyroid still remains a rare site for metastasis. The incidence of metastasis to the thyroid gland varies from 1.2-24% [1], based on various clinical and autopsy studies with most of these cases detected in autopsies. In the recent years, the number of reported cases seems to have increased probably due to the more frequent use of FNAC in any suspected case [2]. The most common sites of primary are breast, bronchi, gastrointestinal system (colon, oesophagus, or stomach) and kidneys [3]. Breast and lung are the two most common sites of primary tumour metastasizing to the thyroid gland according to autopsy studies [4]. While in clinical series, renal cell carcinoma is the most common clinically detected and surgically treated metastatic lesion of the thyroid gland [3].
Metastasis to the thyroid gland indicates end stage disease with poor prognosis. The overall survival of a patient with metastatic thyroid disease depends on the site of primary tumour ranging from 21 to 66 months for breast, kidney and uterine cancer and 1 to 12 months for lung cancer and melanoma [5].
Diagnosis of thyroid metastasis is difficult as not only is it a rare entity but also because most patients are asymptomatic and do not present with any clinically detectable thyroid mass or related symptoms. But now with the advent of imaging and FNAC, more cases are being detected. On ultrasonogram, thyroid metastatic lesions are seen as hyper echoic masses. A CT scan may show calcifications in the thyroid parenchyma, multinodular thyroid enlargement or an isolated nodule. A cold nodule and a hyper metabolic mass are observed in a thyroid scan and a PET scan respectively [6]. Ultrasound guided FNAC is a rapid, minimally invasive, and inexpensive technique for the diagnosis of metastasis to the thyroid gland. However, this technique relies upon the expertise of the performing physician. Also, a close differential diagnosis is anaplastic carcinoma of thyroid which is difficult to differentiate on cytology [5]. IHC can help in confirming metastatic lesions to the thyroid gland. Metastatic lesions are negative for thyroglobulin and calcitonin. TTF-1 has little value as it is positive in both thyroid and lung malignancies. Metastatic renal cell carcinomas are positive for CD10, vimentin and renal cell carcinoma antigen. Estrogen receptor and progesterone receptor can be positive in metastatic breast carcinoma [7]. In this case, PSA and PSAP showed positivity on IHC.
The presence or absence of metastasis is an important prognostic marker for prostatic cancer. The most common sites of metastasis of carcinoma prostate are bone, lung, liver, pleura and adrenal glands [8]. One of the first signs of recurrence or relapse in a patient who has undergone radical prostatectomy or radiotherapy is rise in serum PSA. Metastatic lesions can be confirmed by PET with fluorodeoxyglucose [9].
The largest study on metastasis to the thyroid gland was done by Hegerova L et al., which included 97 cases over a period of 30 years [10]. The most common primary sites included kidney (22%), lung (22%) and head and neck (12%). The most common tumour was clear cell carcinoma of kidney. They concluded that FNAC was a sensitive method to detect metastasis.
Our case is comparable to a case report by Selimoglu H et al., who reported a case of metastatic prostatic cancer to the thyroid in a 73-year-old man [11]. This patient was on hormone ablation therapy for seven years. Radical prostatectomy was not performed. He presented with a palpable mass in the right lobe of thyroid, FNAC of which revealed clusters of atypical cells. IHC revealed these cells to be positive for PSA. As this patient refused surgery, he was kept on close follow-up and was in good condition eight months post FNAC.
Our patient was clinically asymptomatic for two years post prostatectomy. On follow-up, it was found that serum PSA was 3.1 ng/ml which was a rise from previous undetectable levels. PET scan showed a nodule in the thyroid gland, FNAC of which revealed atypical cells. Histopathological examination and IHC studies confirmed metastatic prostatic carcinoma. Post total thyroidectomy, the serum PSA reduced to undetectable levels. There were no postoperative complications. As metastatic prostatic cancer has poor prognosis, he was advised more frequent monitoring of serum PSA which, in his next two checkups, remained undetectable. This is the first case of metastatic tumour to the thyroid gland in our institution. Literature review also indicates a low incidence. PET scan and FNAC studies detect these lesions more frequently and IHC studies help to confirm the diagnosis, all of which were done in our case.
Conclusion
Thyroid gland is an infrequent site for metastasis and even rarer is a primary from the prostate gland. In our case, the clinically asymptomatic patient presented with an incidental solitary lesion in the thyroid gland two years after radical prostatectomy. His serum PSA had increased from undetectable levels to 3.1ng/ml. This thyroid lesion was proven to be metastatic prostatic adenocarcinoma to the thyroid gland, thereby worsening the prognosis of the patient. Hence we would like to stress that any lesion in the thyroid gland, in a patient with history of carcinoma, can be considered as a metastatic lesion, irrespective of his clinical status.
[1]. Papi G, Fadda G, Corsello SM, Corrado S, Rossi ED, Radighieri E, Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experienceClinical Endocrinology 2007 66(4):565-74. [Google Scholar]
[2]. Kim TY, Kim WB, Gong G, Hong SJ, Shong YK, Metastasis to the thyroid diagnosed by fine-needle aspiration biopsyClin Endocrinol 2005 62(2):236-41. [Google Scholar]
[3]. Cichon S, Anielski R, Konturek A, Barczynski M, Cichon W, Metastases to the thyroid gland: seventeen cases operated on in a single clinical centerLangenbeck’s Archives of Surgery 2006 391(6):581-87. [Google Scholar]
[4]. Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA, Metastasis to the thyroid gland. A report of 43 casesCancer 1997 79(3):574-78. [Google Scholar]
[5]. Yang SI, Park K, Kim J, Thyroid metastasis from breast carcinoma accompanied by papillary thyroid carcinomaCase Rep Oncol 2014 7(2):528-33. [Google Scholar]
[6]. Namad T, Wang J, Shipey R, Karim NA, Thyroid metastasis from non small cell lung cancerCase Reports in Oncological Medicine 2013 2013:208213 [Google Scholar]
[7]. Stevens TM, Richards AT, Bewtra C, Sharma P, Tumours metastatic to thyroid neoplasms: a case report and review of the literaturePathology Research International 2011 2011:238693 [Google Scholar]
[8]. Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Metastatic patterns of prostate cancer: An autopsy study of 1,589 patientsHum Pathol 2000 31(5):578-83. [Google Scholar]
[9]. Schöder H, Larson SM, Positron emission tomography for prostate, bladder, and renal cancerSemin Nucl Med 2004 34(4):274-92. [Google Scholar]
[10]. Hegerova L, Griebeler ML, Reynolds JP, Henry MR, Gharib H, Metastasis to the thyroid gland: report of a large series from the Mayo ClinicAm J Clin Oncol 2015 38(4):338-42. [Google Scholar]
[11]. Selimoglu H, Duran C, Saraydaroglu O, Guclu M, Kiyici S, Ersoy C, Prostate cancer metastasis to thyroid glandTumouri 2007 97(3):292-95. [Google Scholar]