BC is the second most common cancer (CA) and one-fifth of all CA among women in India and accounts for 7% of global burden of BC [1]. Fifty percent cases of BC and 38% of deaths related to BC are found in developed countries [2]. BC is a heterogeneous disease encompassing several pathological and molecular subtypes characterized by different outcomes and responses to a given treatment [3].
The combined ER, PR and HER2/neu biomarker expression is a better presentation of the BC status for therapeutic guidance [4]. Much effort is being carried out to identify markers that have biological and therapeutic significance in BC. MUC1, MUC2 and MUC5AC, members of mucin family, are amongst few of the tumour oncoproteins that have demonstrated to be a potential target and are currently under clinical trials [5]. Abnormal mucin expression is recognized to be associated with the development of cancer, cellular growth, differentiation, transformation, adhesion, invasion and immune surveillance [5].
This study was conducted for studying the expression of MUC1, MUC2 and MUC5AC in breast carcinoma cases.
Materials and Methods
This cross-sectional study was conducted in the Department of Pathology in Smt. Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India. Ethical Clearance was obtained. Fifty cases of Primary Breast Carcinoma (PBC) operated upon and diagnosed from 2013 to 2015 were included in the study. The available data for all the patients as regards with age, location of tumour, menopausal status and stage was collected from the records of histopathology section of the department of pathology. Cases in which records/slides/blocks were not available were excluded.
The Modified Radical Mastectomy (MRM) specimens of the test population received were evaluated histopathologically. All the slides were evaluated by two senior histopathologists. The Modified Bloom-Richardson (MBR) system of cancer grading system was used in this study. TNM classification and staging of the cases was done as per American Joint Committee on Cancer (AJCC) guidelines [6].
The most suitable tissue block of PBC was selected for IHC evaluation. A technique of manual tissue array was employed for all the cases subjected for IHC [7]. The primary antibodies used were MUC1 (Clone Ma695; Novocastra), MUC2 (Clone Ccp58; Novacastra), MUC5AC (Clone CLH2; Novacastra), ER (Clone 6F11; Novacastra), PR (Clone PGR312; Novacastra) and Her2/neu (clone CB11, Novacastra). Negative control (without adding primary antibody) was included in all batches. Section from lung and small bowel was used as positive control for MUC1 and MUC5AC. Section from endometrial tissue was used as positive control for ER and PR. Section from BC, which previously showed unequivocal strong immunoreactivity for HER2/neu, was used as positive control for HER2/neu. Sections were examined under High Power Field (HPF) to observe the immunoreactivity.
MUC1 was considered positive if >30% of the tumour cells showed immunoreactivity. MUC2 and MUC5AC were regarded as positive when tumour cells exhibited any amount of immunoreactivity due to their low expression levels [8]. ER and PR were evaluated as per the Allred score and a score of 3 to 8 was considered positive [8]. Scoring of HER2 staining was done according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines and 2+ and 3+ score was regarded as positive [Table/Fig-1] [9].
Photomicrograph showing: a) Circumferential membranous positivity of MUC1 in invasive ductal carcinoma of breast (IHC, 40X); b) MUC2 positivity (IHC, 40X) and; c) MUC5AC positivity in mucinous carcinoma of breast (IHC, 40X); d) strong nuclear ER (IHC, 40X); e) Uniform intense membrane HER2 (IHC, 40X);and f) PR immunoreactivity in tumour cells of IDC of breast. (IHC, 40X).
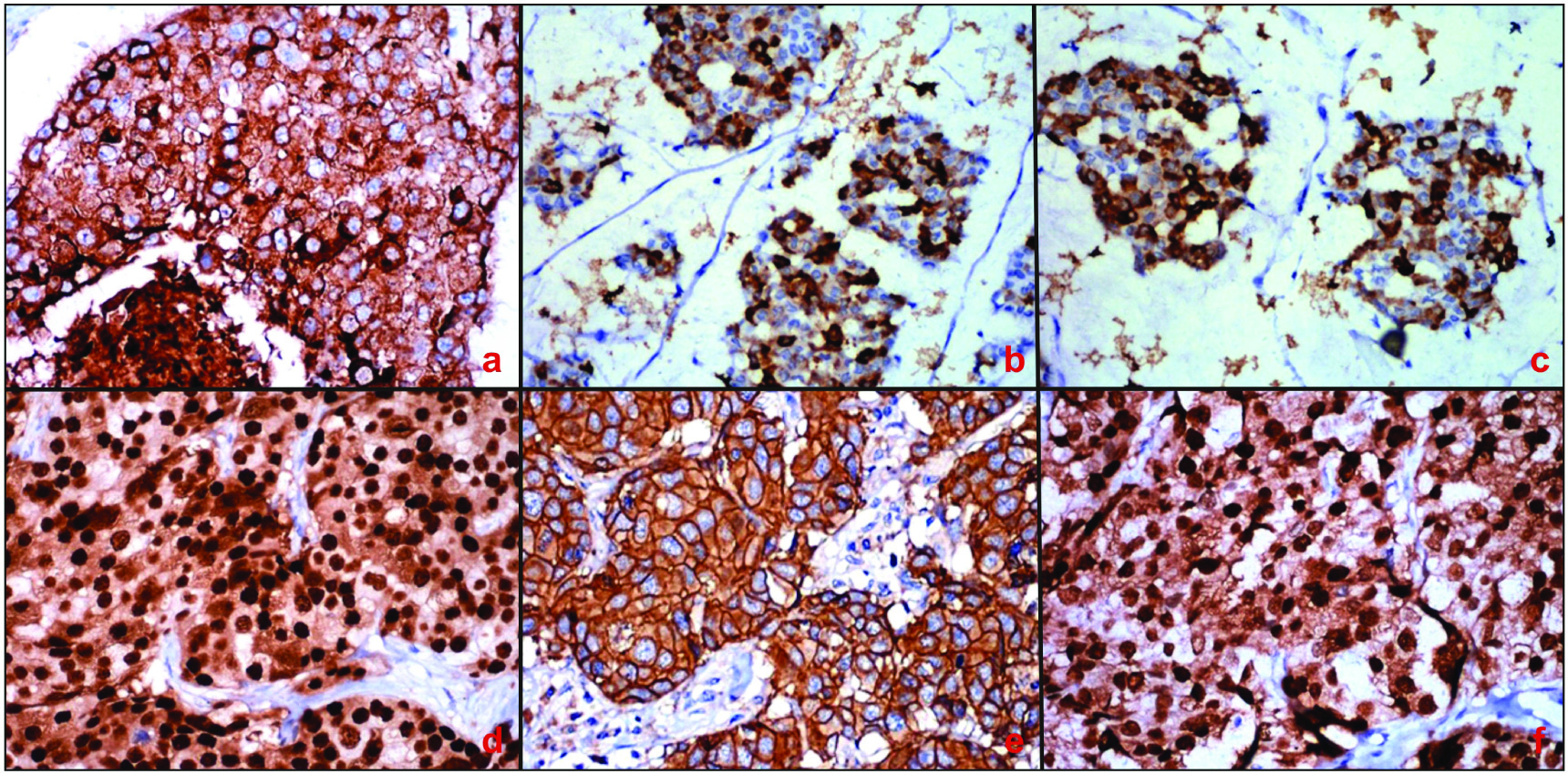
Immunoreactivity for ER and PR was assessed by estimating the percentage of tumour cells showing nuclear staining. More than 10% of the tumour cells showing immunoreactivity were considered as positive [10]. For HER2 moderate to strong complete membrane staining of 10% or more of the tumour cells was considered to be positive [8].
Statistical Analysis
The statistical analysis was done using Primer software and Epi Info Version 7.0 (manufactured by McGraw-Hill) for calculation of interrelationships between the analysed MUC1, MUC2 and MUC5AC expression and clinicopathological variables by Pearson’s Chi-square test and Fisher-exact test. Quantitative data was presented with the help of mean. Qualitative data was presented with the help of frequency and percentage table. The results were considered to be significant when the p-value was less than 0.05.
Results
The various clinicopathological features of PBC are mentioned in [Table/Fig-2]. Out of the 50 cases studied, 23 (46%) cases belonged to Grade II, with maximum number of cases seen in the age group of 41-50 years i.e., 20 (40%) cases. Nineteen (38%) cases belonged to Grade III with maximum number of cases in the age group of 41-50 years and 61-70 years i.e., 8 (42.1%) and 6 (31.6%) cases respectively. Eight (16%) cases belonged to Grade I, out of which 4 (50%) cases belonged to the age group of 61-70 years and 2 cases (25%) in 41-50 years and 51-60 years age group each. However, the association between age of the patient and the grade of the BC was not significant statistically (χ2=8.921; p=0.349). Out of the 50 cases studied, 25 cases (50%) belonged to Stage II. Maximum number of cases i.e., 10 (40%) cases of Stage II BC were seen in the age group of 41-50 as well as 61-70 years. Twenty three (46%) cases belonged to Stage III and majority of cases i.e., 10 (43.5%) cases of Stage III BC were seen in the age group of 41-50 years. However, the association between age of the patient and the stage of BC was not significant statistically (χ2=7.226; p=0.512).
Clinicopathological characteristics of 50 cases.
| Clinicopathological characteristics of 50 cases | Number of tumours (%) |
|---|
| Laterality | |
| Right | 23(46) |
| Left | 27(54) |
| Menopausal status |
| Premenopausal | 12(24) |
| Post-menopausal | 38(76) |
| Histopathological types |
| IDC (NOS) | 46(92) |
| IDC+mucinous features | 2(4) |
| mucinous (colloid) carcinoma | 2(4) |
| Status of lymphovascular invasion |
| Present | 41(82) |
| Absent | 9(18) |
| Tumour Size (pT) |
| pT1 | 4(8) |
| pT2 | 28(56) |
| pT3 | 10(20) |
| pT4 | 8(16) |
| ALN metastasis |
| N0 | 17(34) |
| N1 | 16(32) |
| N2 | 11(22) |
| N3 | 6(12) |
| MBR Grade |
| Grade I | 8(16) |
| Grade II | 23(46) |
| Grade III | 19(38) |
| Stage |
| I | 2(4) |
| II | 25(50) |
| III | 23(46) |
| IHC markers in PBC |
| MUC1 | 29(58) |
| MUC2 | 4(8) |
| MUC5AC | 3(6) |
| ER | 24(48) |
| PR | 18(36) |
| HER2/neu | 32(64) |
IDC-NOS=infiltrating duct carcinoma not otherwise specified, MBR=modified Bloom Richardson grading, IHC=immunohistochemistry, PBC=primary breast carcinoma
Expression of MUC1, MUC2 and MUC5AC in BC cases [Table/Fig-2] and with various clinicopathological features is shown in [Table/Fig-3]. MUC1 overexpression demonstrated statistically significant association with positive ER and PR expression. MUC2 overexpression demonstrated statistically significant association with positive ER expression. MUC2 and MUC5AC overexpression showed a significant association with histologic grade. No significant association was seen between MUC1, MUC2, MUC5AC expression and other clinicopathological parameters.
MUC1, MUC2 and MUC5AC expression and clinicopathological parameters of breast carcinoma.
| Variables | MUC1 | MUC2 | MUC5AC |
|---|
| Pos | Neg | p-value | Pos | Neg | p-value | Pos | Neg | p-value |
|---|
| Histologic grade | I | 7 | 1 | χ2=3.753; p=0.153 | 3 | 5 | p<0.05 | 3 | 5 | p<0.01 |
| II | 14 | 9 | 1 | 22 | 0 | 23 |
| III | 9 | 10 | 0 | 19 | 0 | 19 |
| Lymphovascular invasion | Present | 22 | 19 | χ2=0.911; p=0.340 | 3 | 38 | p=0.560 | 2 | 39 | p=0.456 |
| Absent | 7 | 2 | 1 | 8 | 1 | 8 |
| Nodal Stage | 0 | 8 | 9 | χ2=4.376; p=0.299 | 3 | 14 | χ2=5.110; p=0.218 | 2 | 15 | χ2=2.592; p=0.625 |
| 1 | 12 | 4 | 0 | 16 | 0 | 16 |
| 2 | 7 | 4 | 0 | 11 | 1 | 10 |
| 3 | 2 | 4 | 1 | 5 | 0 | 6 |
| ER | Pos | 18 | 6 | p<0.05 | 4 | 20 | p<0.05 | 0 | 24 | p=0.236 |
| Neg | 11 | 15 | 0 | 26 | 3 | 23 |
| PR | Pos | 14 | 4 | p<0.05 | 3 | 15 | p=0.127 | 0 | 18 | p=0.544 |
| Neg | 15 | 17 | 1 | 31 | 3 | 29 |
| Her2/neu Receptor | Pos | 19 | 13 | χ2=0.001; p=0.971 | 2 | 30 | p=0.612 | 3 | 29 | p=0.544 |
| Neg | 10 | 8 | 2 | 16 | 0 | 18 |
| Tumour size | T1 | 3 | 1 | χ2=0.992; p=1 | 1 | 3 | χ2=4.969; p=0.232 | 0 | 4 | χ2=1.114; p=1 |
| T2 | 15 | 14 | 1 | 27 | 2 | 26 |
| T3 | 6 | 4 | 2 | 8 | 1 | 9 |
| T4 | 5 | 3 | 0 | 8 | 0 | 8 |
| Triple negative | Pos | 1 | 5 | χ2=3.048; p=0.081 | 0 | 6 | p=1 | 0 | 6 | p=1 |
| Neg | 28 | 16 | 4 | 40 | 3 | 41 |
| Stage | I | 1 | 1 | χ2=0.114; p=0.944 | 1 | 1 | χ2=5.210; p=0.074 | 0 | 2 | χ2=0.416; p=0.812 |
| II | 15 | 10 | 2 | 23 | 2 | 23 |
| III | 13 | 10 | 1 | 22 | 1 | 22 |
Pos=positive, Neg=Negative, ER= Estrogen Receptor, PR= Progesterone Receptor
Chi-square test, Fisher-exact test applied
Discussion
In present study, 58% cases exhibited MUC1 positivity. Studies in literature have reported more than 70% MUC1 positivity [Table/Fig-4] [5,8,10-14]. Most of the tumours in the present study were of higher stage and grade. In our study, MUC1 positivity was higher in stage 2 and 3 tumours (30% & 26%) though there was no statistically significant difference (χ2=0.114; p=0.944). No study describing MUC1 expression and stage of tumour was found in literature. There was no significant association between MUC1 expression and tumour size as observed in the studies done by Do SI et al., and Pereira MB et al., [8,12]. This was in contradiction to the previous study done by Rakha EA et al., who found that MUC1 expressing tumour were smaller in size compared to MUC1 non-expressing tumours [5]. Few BC cases (8%, 4/50) included in the present study were of T1. Our study showed MUC1 positivity in 16% and 24% cases of N0 and N1stage. We did not find any significant association between MUC1 expression and nodal stage (χ2=4.376; p=0.299). Similar findings were seen in the study done by Do SI et al., of 240 BC cases with MUC1 positivity in 31% and 27% of N0 and N1 stage [8]. Do SI et al., (p=0.816) and Pereira MB et al., also found no correlation between MUC1 expression and nodal stage [8,12]. Contrary to this, Rakha EA et al., found a borderline significance of MUC1 expression with the presence of lymph node metastasis (p=0.08) [5].
Percentage of positive cases of MUC1, MUC2 and MUC5AC in different studies and our study.
| Study | Number of cases | MUC1 (%) | MUC2 (%) | MUC5AC (%) |
|---|
| Rakha EA et al., [5] | 1447 | 91 | 8.3 | 37 |
| Do SI et al., [8] | 240 | 93.6 | 6.2 | 4.8 |
| Lau SA et al., [10] | 14 | 100 | 7.1 | 0 |
| Walsh MD et al., [11] | 210 | - | 19 | - |
| Pereira MB et al., [12] | 68 | 73.5 | - | 7.3 |
| Rahn JJ et al., [13] | 71 | 76.1 | - | - |
| Elseed SMH et al., [14] | 40 | 72.5 | - | - |
| Present study | 50 | 58 | 8 | 6 |
We found a significant positive correlation between MUC1 positivity and ER, PR expression (p<0.05) as seen in the previous studies [5,8,15,16]. No correlation was observed between MUC1 and Her2 expression in our study as seen in literature [8,12]. MUC1 expressing tumours were found to be associated with increased ER, PR expression indicating correlation with functional differentiation of tumour.
In present study, no significance was noted between triple negative BC and MUC1 expression (p=0.081) as seen in study done by Elseed SMH et al., [14]. Only six cases of triple negative BC were included in the present study. A study with larger sample size may be needed to elucidate the matter further. Unlike this, Do SI et al., noted a statistically significant association between triple negative BC and MUC1 expression (p<0.001) [8].
No significant association was noticed between MUC1 and LVI (p=0.340) as seen in study done by Do SI et al., (p=0.860) [8].
Though in our study, there was no significant association between MUC1 positivity and histopathological grade (p=0.153), it was observed that MUC1 negative tumours were associated with higher histologic grade. This finding was consistent with previous studies by Rakha EA et al., Vegt B et al., and Luna-More S et al., who stated that negative MUC1 was associated with higher tumour grades and poor prognosis [5,15,16].
About 8% (4/50) of the cases showed MUC2 expression in the present study as seen in literature [Table/Fig-3]. Though there was no significant correlation between MUC2 expression and stage of tumour, it was noted that MUC2 negative tumours were associated with higher stage. This suggests that negative MUC2 expression in BC is associated with aggressive tumour behaviour. Such correlation between MUC2 expression and stage of tumour was not found in literature.
In the present study, no significant association was noted in MUC2 expression and tumour size. Similar results were obtained by Rakha EA et al., [5]. This was in contrary to the finding observed in the study done by Walsh MB et al., who found that MUC2 expression was present in higher proportion of tumours of less than 1cm and greater than 5 cm [11].
MUC2 positivity of 6% in N0 stage was noted in the present study. We did not find any significant association between nodal stage and MUC2 expression (p=0.218). Similarly no association was noted in the studies done by Do SI et al., (p=0.873) and Walsh MD et al., [8,11]. Contrary to this, Rakha EA et al., found a significant correlation of MUC2 expression with the presence of lymph node metastasis (p=0.034) [5].
In our series we found significant statistical correlation between MUC 2 expression and ER positivity (p<0.05). However, there was no significant correlation between MUC2 positivity with PR expression (p=0.127). Our findings were consistent with the previous studies done by Do SI et al., (p=0.556 and p=0.902) and Walsh MD et al., [8,11]. In a study by Rakha EA et al., ER showed a highly significant association with MUC2 expression (p<0.001) [5]. There was no correlation of HER2 expression with MUC2 expression in our study. Similar finding was noticed by Do SI et al., (p=1) [8].
In our study no significance was noted between triple negative BC and MUC2 expression (p=1). This finding was consistent with the study done by Do SI et al., who found no significant association between triple negative BC and MUC2 expression (p=0.473) [8]. No significant association was noticed between MUC2 expression and Lymphovascular Invasion (LVI) (p=0.560). This finding was contradictory to the previous study done by Rakha et al., who found an inverse association of MUC2 expression with LVI (p=0.860) [5].
In our study, there was a significant correlation between the MUC2 expression and histopathological grade (p<0.05), which suggests that MUC2 negative tumours were associated with higher tumour grade. This finding states that negative MUC2 expression in BC is associated with aggressive tumour behaviour. In contradiction to this, Rakha EA et al., Do SI et al., and Walsh MD et al., found no significant association between MUC2 expression and histologic grade [5,8,11].
In this study, MUC5AC was detected only in 6% of the cases which is in agreement with other studies such as Do SI et al., and Pereira MB et al., but contrary to Rakha EA et al., who found MUC5AC expression in 37% of the cases [5,8,12].
There was no statistical correlation of MUC5AC positivity with respect to stage. However, it was noted that MUC5AC negative tumours were associated with higher tumour stage. This states that negative MUC5AC expression in BC is associated with aggressive tumour behaviour. No correlation between MUC5AC expression and stage of tumour was found in literature.
In the present study no significant association was noted in MUC5AC expression and tumour size. Similar results were obtained by Rakha EA et al., and Pereira MB et al., [5,12].
Our study showed MUC5AC positivity of only 4% in N0 stage. We did not find any significant association between nodal stage and MUC5AC expression (χ2=2.592; p=0.625). Similarly no association were noted in the studies done by Rakha EA et al., Do SI et al., (p=0.962) and Pereira MB et al., [5,8,12].
In our series we found no significant correlation between MUC5AC positivity with ER, PR and HER2. These findings were consistent with the previous studies done by Rakha EA et al., Do SI et al., and Pereira MB et al., [5,8,12].
In present study no significance was noted between triple negative BC and MUC5AC expression (p=1). This finding was consistent with the study done by Do SI et al., who found no significant association between triple negative BC and MUC5AC (p=1) [8].
No significant association was noticed between MUC5AC expression and LVI (p=0.456), however, it was noted that MUC5AC negative tumours showed higher frequency of LVI. This suggests a possibility that MUC5AC mucin could serve as an obstruction hindering the spread of tumour cells explaining the better prognosis usually seen in mucinous carcinomas of the breast. Rakha EA et al., and Do SI et al., found no association of MUC5AC expression with LVI [5,8].
In our study, there was a high statistically significant correlation between MUC5AC positivity and histopathological grade (p<0.01) which suggests that, MUC5AC negative tumours were associated with higher tumour grade. This finding states that MUC5AC expression in BC is associated with aggressive tumour behaviour. This was in contrary to Rakha EA et al., Do SI et al., and Pereira MB et al., who found no significant association between MUC5AC expression and histologic grade [5,8,12].
Limitation
Variations in the findings of the current and previous studies may relate to differences in immunohistochemical protocols, antibodies used, scoring systems, area of the tumour examined and the sample size.
Conclusion
The positivity for MUC1, MUC2 and MUC5AC in BC was 58%, 8% and 6% and for ER, PR and HER2 was 48%, 36% and 64% respectively. High MUC1 expressing tumours were found to be more frequently ER and PR positive. There was a statistically significant correlation between MUC2 expression and ER positivity. It was observed that MUC1, MUC2 and MUC5AC negative tumours were associated with higher histologic grade with statistically significant association for MUC2 and MUC5AC. It was noted that MUC2 and MUC5AC negative tumours were associated with higher tumour stage and thus with aggressive tumour behaviour. MUC5AC negative tumours showed higher frequency of LVI.
IDC-NOS=infiltrating duct carcinoma not otherwise specified, MBR=modified Bloom Richardson grading, IHC=immunohistochemistry, PBC=primary breast carcinoma
Pos=positive, Neg=Negative, ER= Estrogen Receptor, PR= Progesterone Receptor
Chi-square test, Fisher-exact test applied