White opaque localized tooth discolorations which are the precursors of frank enamel caries can result from a number of factors and are usually a concern of aesthetics for patients. The treatment of such lesions should aim upon both improving the aesthetics and prevention of caries progression. White Spot Lesions (WSL) are usually formed in patients who have undergone fixed orthodontic treatment which makes daily oral hygiene maintenance difficult and increases the risk of enamel demineralization and are frequently called as orthodontic scars [1]. Other factors like xerostomia, high caries index, fluorosis and developmental hypoplasia also lead to the formation of white spot lesions [2].
Early enamel lesions present an apparently intact outer layer, followed by a subsurface porous area, called the body of the lesion. The microporosities of enamel carious lesions are filled with either a watery medium or air. Due to the difference between the refractive index of sound enamel (RI:1.62) and water (RI:1.33) or air (RI:1.0) [3] within the body of the lesion, ambient light which shines on the teeth is deflected and scattered, making the initial caries lesion appear as a clinically visible opacity especially when they are dessicated [4]. The polycrystalline structure of hypomineralized enamel is more porous and disorganized than normal enamel with a reported 28% reduction in mineral content, 80% more carbonated apatite and 3 to 15 fold elevation in protein content. Thus, the hardness of hypomineralized enamel is significantly lower than sound enamel [5].
Different remineralization strategies have been reported using bioactive glass, fluoride releasing materials, Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) complexes, calcium hydroxide and portland cement, some of which are capable of restoring partially demineralized enamel. However, these treatment options have limitations such as; they do not give immediate results, require patient compliance, and stains from external sources may get incorporated into the lesions during remineralization. Besides, remineralization occurs only superficially, while the body of the lesion remains porous, which explains the unpredictable results and persistence of whitish discoloration [6].
A promising intermediary treatment option between preventive and restorative therapy for the arrest of caries lesion is the infiltration of low-viscosity light-curing resin into the subsurface lesion. As the porosities of enamel caries act as diffusion pathways for acids and dissolved minerals, infiltration of these lesions with resin might occlude the pathways, leading to the arrest of caries progression [7]. Besides, the microporosities filled with the resin (RI:1.46) cannot evaporate and the aesthetic improvement is achieved instantly [8]. However, long term colour stability with this technique is questionable and microleakage due to polymerization shrinkage of low viscosity resin can occur leading to progression of caries [9]. On the other hand, colloidal silica nanoparticles when tested upon fully demineralized dentin showed the highest remineralization potential, restoring upto 20% of the phosphate levels of sound dentin and demonstrating a 16% recovery of the mineral volume [10,11].
Microhardness testing of demineralized and infiltrated lesion is a reliable method for obtaining indirect information about mineral content changes of dental hard tissues. Penetration depth is a parameter where the penetration of a material into enamel surface or dentinal tubules is studied. Hence, the aim of the study was to evaluate and compare the surface microhardness and penetration depth of a low viscosity resin and colloidal silica nanoparticle infiltrates into artificially created white spot lesions. The hypothesis tested was there will be no difference in the surface microhardness and penetration depth between the tested infiltrants.
Materials and Methods
The present in vitro study was carried out in the Department of Conservative Dentistry and Endodontics, GITAM Dental College and Hospital, Visakhapatnam, Andhra Pradesh, India, in a span of 12 weeks. Forty maxillary central incisors of similar dimensions, free of cracks and defects, extracted due to periodontal reasons were collected and stored in thymol solution until use. The teeth were thoroughly cleaned using pumice slurry and a prophylaxis brush in a contra-angled handpiece and were decoronated at approximately 1 mm coronal to the cemento-enamel junction. Then, the crowns were embedded in acrylic resin blocks of 1 inch diameter and 1.5 cm height, so that the labial surfaces of crowns were exposed and were parallel to the floor, as flat surfaces are required for microhardness testing.
Surface Microhardness Testing
The surface microhardness was measured at baseline using a Vicker’s microhardness tester (400 Series, Wilson Wolpert, Germany) with a diamond indenter fitted with a 300 g load. Three indentations (500 μm apart) were made on the middle third of the labial surfaces of the crowns with a dwell time of 15 seconds. Digital readings were noted for each indentation and average was taken for each sample.
To create artificial white spot lesions, the specimens were immersed in a freshly prepared demineralizing solution composed of 2.2 mM calcium chloride, 2.2 mM monopotassium phosphate and 0.05 mM acetic acid having pH adjusted to 4.4 using 1 M potassium hydroxide for 96 hours. After washing with distilled water and drying, all the samples were subjected to post-demineralization microhardness testing. Then, the samples were randomly allocated into two groups (n=20 each) according to the infiltrant used [Table/Fig-1].
Infiltrants used in the study
| Material | Composition |
|---|
| Resin Infiltration Kit (ICON DMG, Hamburg, Germany) | Icon Etch: 15% Hydrochloric acid, pyrogenic silicic acid, surface active substances.Icon Dry: 99% EthanolIcon Infiltrant: Tetraethylene glycol dimethacrylate, initiators, additives. |
| Colloidal Silica infiltrant (Arrow Fine Chemicals, Rajkot, Gujarat) | Silica (SiO2): 29.6 wt% suspension in waterpH at 25oC: 10Average particle diameter: 8.3 nmStabilizing counter ion: Sodium |
In Group 1, the demineralized labial surfaces of the specimens were treated using the Icon- smooth surface kit (Icon, DMG, Hamburg, Germany) according to the manufacturer’s instructions. Lesions were etched with 15% Hydrochloric acid gel (Icon Etch) for two minutes and washed with water spray for 30 seconds. After drying with 99% ethanol (Icon Dry) for 30 seconds, lesions were stained with 0.1% ethanolic solution of tetramethyl rhodamine isothiocyanate dye (Macsen Labs Pvt. Ltd. Udaipur, Rajasthan, India) for 12 hours. Teeth were dried with compressed air for 10 seconds and the infiltrant (Icon- Infiltrant) was applied onto the surface for 3 minutes using applicator with occasional agitation. After light curing (Bluephase C8 LED light curing unit, Ivoclar Vivadent, USA) for 40 seconds, the application of infiltrant was repeated for one minute and cured to compensate for the polymerization shrinkage. Finally, the infiltrated surfaces were polished at slow speed using sof-lex finishing and polishing kit (3M ESPE, Minnesota, US) to remove surface irregularities.
After demineralization procedure, the samples in Group 2 were directly immersed in 0.1% ethanolic solution of tetramethyl rhodamine isothiocyanate for 12 hours and air dried for 10 seconds. Then, the specimens were immersed in chambers containing 10 ml of 29.6% colloidal silica suspension (Arrow Fine Chemicals, Rajkot, Gujarat, India) for 24 hours and were allowed to dry and polished. All the samples (n=40) were again subjected to post treatment microhardness testing.
Penetration Depth Measurement
The specimens were cut perpendicular to the surface across the lesions to obtain slices of 300 μm thickness, using a hard tissue microtome (Leica SP 1600, Leica biosystems, Germany). To bleach all the red fluorophore that has not been enclosed by the infiltrant (resin or silica), each section was stored in individual vials containing 30% hydrogen peroxide solution (Merck Life Science Pvt. Ltd. Vikhroli, Mumbai) for 12 hours at 37°C. The sections were then washed with water for 60 seconds and allowed to dry. Subsequently, specimens were observed under confocal laser fluorescence microscope (LSM 880, Zeiss, Germany) under 10X magnification to measure the lesion depth and penetration depth of the infiltrant, using software (LSM Image browser, Zeiss, USA).
Under CLFM, the lesion appeared darkened and the infiltrated material appeared red. To achieve reproducible measurements, three deepest measurements (in microns) for both lesion and infiltrant depth were taken for each section and their averages were calculated as mean of maximum lesion depth (LDmax) and maximum penetration depth (PDmax). As the main outcome, the percentage of penetration (PP) was calculated using the formula [12];
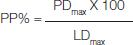
Statistical Analysis
Collected data was subjected to statistical analysis using SPSS software version 22.0 Assumption of normal distribution was checked using Kolmogorov-Smirnov and Shapiro-Wilks tests. To compare the mean values of microhardness and penetration depth between groups, independent samples t-test was applied and to compare the microhardness at different time points, paired t-test was used. Significance level was fixed at 5% (α = 0.05).
Results
The surface microhardness values greatly decreased from baseline to post-demineralization. Both the groups showed increase in microhardness after treatment. Paired samples t-test revealed that there was no significant difference between the groups at baseline and after demineralization [Table/Fig-2]. But, significant difference was observed after treatment (p<0.001) showing highest values for resin infiltration group.
Comparison of mean surface microhardness (VHN) values using independent sample t-test.
| Variables | Group | N | Mean | Std. Dev | t-value | p-value |
|---|
| Baseline | Resin Infiltration | 20 | 118.79 | 11.81 | 0.082 | 0.935 |
| Colloidal Silica Infiltration | 20 | 118.50 | 10.20 |
| Post-demineralization | Resin Infiltration | 20 | 75.63 | 7.85 | 0.104 | 0.918 |
| Colloidal Silica Infiltration | 20 | 75.37 | 8.33 |
| Post treatment | Resin Infiltration | 20 | 101.77 | 9.02 | 5.453 | <0.001 |
| Colloidal Silica Infiltration | 20 | 86.76 | 8.37 |
p=<0.05
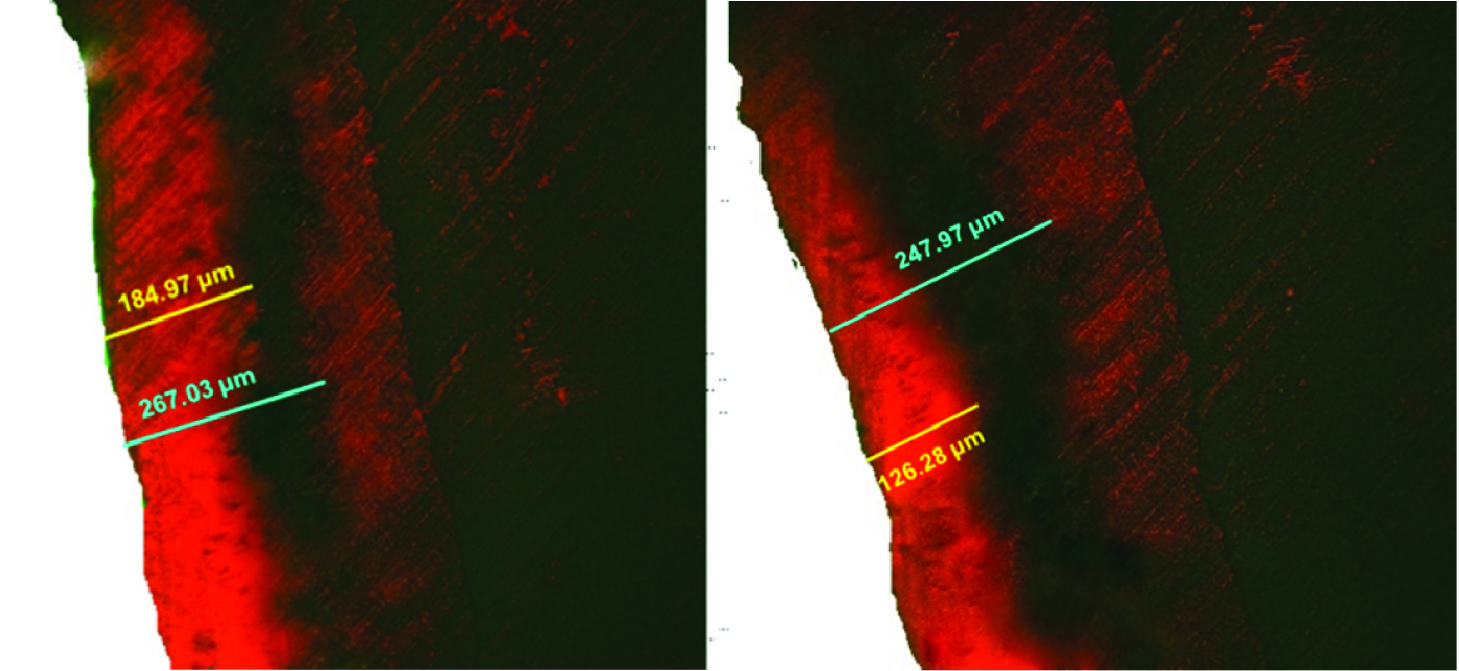
Independent sample t-test [Table/Fig-3] revealed significant difference in the penetration depth and the percentage of penetration between the groups (p<0.001). The resin penetration depth (67.14%) was shown to be greater compared to colloidal silica (53.54%). It was evident from the confocal images obtained, that the red fluorescence covered larger area in samples treated with resin compared to colloidal silica [Table/Fig-4]. This observation indicates that penetration depth of resin was deeper and more uniform than colloidal silica infiltrate.
Independent samples t-test to compare mean percentage penetration values between groups.
| Variables | Group | N | Mean | Std. Dev | t-value | p-value |
|---|
| Percentage penetration | Resin infiltration | 20 | 67.14 | 1.78 | 21.229 | <0.001 |
| Colloidal silica infiltration | 20 | 53.54 | 2.23 |
p=<0.05
Confocal laser microscopic images for penetration depth.
A) Group 1 - resin infiltration B) Group 2 - Colloidal silica infiltration (Blue line indicates the lesion depth; yellow line indicates the penetration depth of infiltrant.)
Discussion
The main goal of treating white spot lesions is to arrest their progression and to improve the aesthetics by increasing the translucency of the opaque lesions [13]. Caries infiltration is a technique that arrests the lesion progression by occluding the microporosities which provide diffusion pathways for acids and dissolved minerals. It is a simple and micro-invasive approach for the treatment of lesions which have not progressed enough to require invasive restorative therapy [8].
In early carious lesions that are too advanced for fluoride therapy, resin infiltration has shown inhibition of caries progression [14]. The resin used in this technique shows very low viscosity, low contact angles to enamel and high surface tension, that penetrates into the lesion by capillary forces [15]. The use of 15% hydrochloric acid for etching the surface layer has been shown to be effective and allows deeper infiltration of the resin into the body of the lesion [16]. The use of solvents such as ethanol, acetone and water in resin infiltrates show lower surface tension, viscosities and high penetration coefficient compared with materials without solvents [17]. It has been reported that, irrespective of the seal achieved by resin infiltration, microleakage due to polymerization shrinkage could lead to progression of caries [9]. Thus, the efficacy of caries infiltration treatment depends mainly on the penetration of infiltrant upto the depth of the lesion and not by improving the translucency of the lesion.
Colloidal silica nanoparticles act as a scaffold for the formation of hydroxyapatite crystals and mineralization of the dentin collagen matrix under higher pH exhibiting mineralization potential. The nanoparticles in the inter and intra-fibrillar collagen spaces reduced the energy barrier, inducing the formation of cluster of inorganic ions. The formation of these inorganic ions was further enhanced by immersing the demineralized specimens in artificial saliva [10]. Besinis A et al., found that colloidal silica nanoparticles having small particle size (12 nm) showed greater penetration and remineralization when compared to nano-hydroxyapatite. The use of acetone as a vehicle is reported to enhance the infiltration capacity of sol-gel nanoparticles. It is said that smaller the particle size, greater the infiltrative capacity. They stated that, under Transmission Electron Microscope (TEM) examination, the silica particles penetrated the dentin tissue and remained in place without precipitation [11].
Colloidal silica has shown greater infiltrative and remineralizing capacity when applied to demineralized dentin [10,11]. Resin infiltration has been extensively studied for its infiltrative potential and proven to be quite effective in arresting progress of early carious lesions. Therefore, we chose to compare it with resin infiltration for its penetration depth and surface microhardness in artificial enamel carious lesions.
The decrease in microhardness after demineralization was reported, as the process softens the enamel by chemical dissolution of enamel rods and creates voids. Microhardness was greater in samples treated with resin infiltration correlating with the previous study results [18]. The hardness, tensile strength, flexural strength, and fracture toughness of resins increases with the degree of conversion of double bonds [19]. This reflects the ability of the low viscosity resin to fill the spaces between the remaining crystals of the porous lesion, creating a diffusion barrier not only at the surface, but also within the enamel lesion body and thus the demineralized tissues reharden, improving the mechanical strength. The findings of the present study were similar to those reported by Torres CRG et al., and Parisv S et al., in which the microhardness of carious lesions were significantly increased with resin infiltration when compared with untreated artificial lesions after demineralization [18,20]. Taher NM et al., also presented that, enamel surfaces treated with an infiltrant showed significantly higher surface hardness when compared to a fissure sealant [7].
The mean lesion depth formed by the demineralizing process of all the specimens was about 265 uM. This indicates that the demineralization protocol was accurate and the lesions formed were of uniform depth. The penetration depth of resin infiltrant was significantly greater than colloidal silica in this study. In a similar study, resin infiltration showed better penetration depth and microhardness when compared to remineralizing agents such as CPP-ACP and fluoride releasing adhesives [21]. Paris S et al., showed that pit and fissure caries lesions when treated with an infiltrant showed significantly higher penetration depth than treatment with sealant [22]. Resin being a low viscosity flowable material penetrates deeper into the enamel microporosities by the capillary action, whereas, the colloidal silica nanoparticles with 8.3 nm particle diameter have the property of maintaining their size and shape and remain in place after drying. So, the silica particles might have been lodged in the enamel voids and sealed the porous structures improving the microhardness of the enamel, but are not able to penetrate into the voids with diameter smaller than the particle size. Based on the results of previous studies [9,10], we anticipated improved microhardness nearing to the baseline values with colloidal silica infiltration. Lower microhardness values resulted in this study might be due to compositional difference of colloidal silica used, from the previous study. Summary of the studies related to microhardness and penetration depth of remineralizing agents have been presented in [Table/Fig-5].
Studies related to microhardness and penetration depth of remineralization agents [10-12, 14, 17, 23-27].
| Authors | Remineralizing agents used | Study design | Remarks/Results |
|---|
| Paris S et al., [17] | Five adhesives, fissure sealant and 66 experimental composites | In vitroPenetration coefficients were determined | Highest penetration coefficients were found for agents containing Triethylene glycol dimethacrylate (TEGDMA), 2-hydroxyethyl methacrylate (HEMA) and 20% ethanol. |
| Shibata Y et al., [23] | Colloidal hydroxyapatite and Beta-tricalcium phosphate | In vitroMicromechanical properties | Beta tricalcium phosphate better than hydroxyapatite |
| Kielbassa et al., [14] | Resin infiltration(RI) | Review | Resin infiltration combined with remineralizing agents can significantly reduce caries progression. |
| Liu Y et al., [24] | Adhesive and resin infiltration | In vitroPenetration depth by Confocal Laser Scanning Microscopy | Resin infiltration penetrated lesion completely. |
| Paris S et al., [12] | Resin infiltration at different application times | In vitroPenetration depth by Confocal Laser Scanning Microscopy | Natural non-cavitated proximal lesionsin primary molars were deeply infiltrated afterone-min application in vitro. For deeper lesions,more consistent results were obtained after3 min. |
| Besinis A et al., [10] | Silica and hydroxyapatite nanoparticles | In vitroEffectiveness and penetration depth by scanning electron microscopy, trasmission electron microscopy and Energy dispersive X-ray spectroscopy | Silica showed better infiltration |
| Milly H et al., [25] | BAG and polyacrylic acid modified BAG (PAA-BAG) | In vitroSurface microhardness and penetration depth | BAG and PAA-BAG surface treatments both enhance enamel white spot lesion remineralization |
| Besinis A et al., [11] | Silica and hydroxyapatite nanoparticles (NPs) | In vitroRemineralizing potential | Demineralized dentin infiltrated with silica NPs causes heterogeneous mineralization of collagen matrix following exposure to an artificial saliva solution. |
| Meyer -Leuckel H et al., [26] | Resin infiltration vs non-invasive and oral hygiene measures (flossing, fluoride application) | Randomized split-mouth, placebo-controlled clinical trial | Resin infiltration is more efficacious in reducing lesion progression compared with individualized noninvasive measures alone |
| Kumar H et al., [27] | Resin infiltration in hypomineralized enamel | In vitroSurface microhardness | Resin infiltration did not increase microhardness |
The limitations of this study are, the staining protocol was modified to study the penetration of colloidal silica under confocal microscope. The colloidal silica suspension used in the present study slightly differs in composition from the one used in previous studies. The samples in those studies were immersed in colloidal silica suspension for 24 hours which is not clinically applicable and also differs from the application method of resin infiltration. Apart from that, in related previous studies [10,11], after colloidal nanosilica infiltration, the specimens were exposed to artificial saliva. It was hypothesized that the remineralizing potential of dentin is significantly slower than that of enamel due to reduced amount of phosphate and nucleating mineral and the presence of inhibitory non-collagenous protein [11]. It was suggested that the phosphate binds to the silica nanoparticles and enhances remineralization, when combined with ionic calcium, which is also available in artificial saliva. This protocol was not followed in the present study that might be one of the reasons for lower hardness values of colloidal silica when compared to resin infiltration.
Further studies need to be conducted to understand the properties and remineralizing potential of colloidal silica nanoparticles and how they can be clinically utilized in arresting caries progression and improving aesthetics.
Conclusion
On the basis of the results of the present in vitro study, the null hypothesis was rejected and it can be concluded that both surface microhardness and penetration depth of resin infiltration was significantly greater than colloidal silica infiltration.
p=<0.05
p=<0.05