A proper knowledge of the anatomy of the furcation area as well as the tooth morphology is very essential for initiating furcation therapy. The main objective of furcation treatment is elimination of microbial plaque and establishment of a proper tooth contour to facilitate self performed plaque control. A wide array of bone graft materials, are available to treat furcations. Superior results have been reported when bone grafts are used along with GTR membranes, however, this treatment modality is not economically feasible to all the patients [1,2]. This increased the scope for the use of platelets and its derivatives. Choukron J et al., developed PRF which can be used both as a graft and a membrane [3]. PRF consists of a fibrin meshwork within which cytokines and glycoproteins are found. The biochemical constituents within PRF have been observed to help in healing of periodontal defects. Moreover, it has been observed that PRF acts as a matrix for the growth of periosteal cells which favour bone repair [3]. Its use as a membrane also, has been advocated because PRF membranes have the ability to augment soft tissue healing by protecting the site of surgery. It has been observed that the membrane acts a biomimetic connector to the graft and acts as a scaffold to promote the growth of new blood vessels, and facilitate the growth of osteoprogenitor cells to the centre of the graft [4].
Hence, the present study was designed to estimate the effectiveness of PRF as a graft and a membrane in comparison to allograft and GTR in Grade II mandibular molar furcation defects.
Materials and Methods
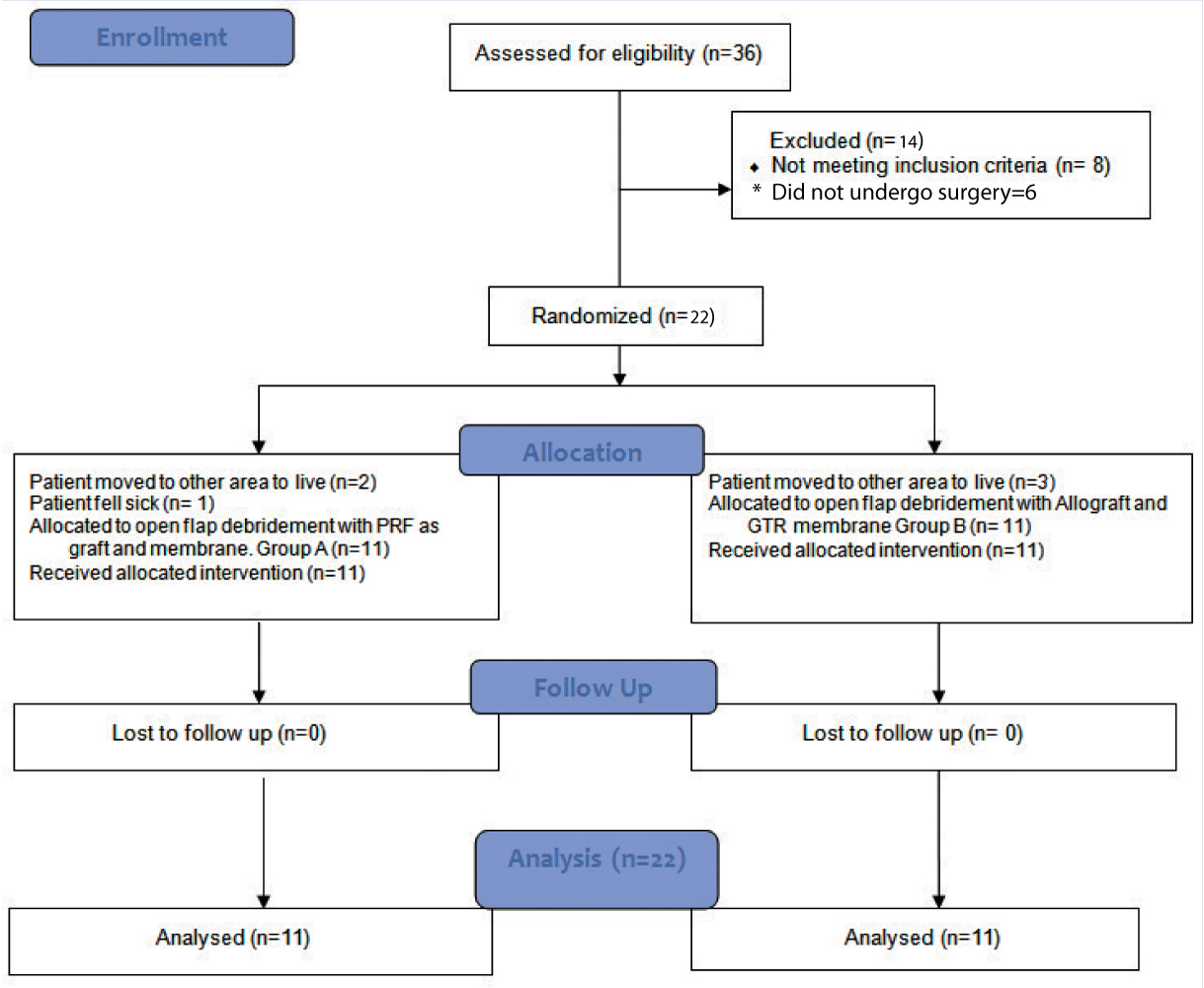
This prospective, randomized, parallel arm, interventional trial which was conducted in the outpatient wing of the Department of Periodontics, Panineeya Mahavidhyalaya Institute of Dental Sciences in Hyderabad, India. The guidelines of Helsinki declaration of 1975 as revised in 2000, were strictly followed while conducting the study. This study was approved by the Institutional Review Board and registered in clinical trials registry (NCT 02430519). A written informed consent was signed by each patient before the start of the study. The trial was conducted from September 2012 to May 2013. Thirty six subjects with Grade II mandibular buccal furcal defects were screened for the study, out of which 14 subjects were excluded (eight patients did not meet inclusion criteria and six subjects did not undergo surgery due to various reasons). Study sample consisted of 22 subjects (14 males and eight females) aged between 30 and 50 years [Table/Fig-1].
CONSORT patient flow chart.
Selection Criteria
Systemically healthy subjects aged between 30-50 years, having a probing depth ≥ 5 mm and Grade II furcation involvement in one of their mandibular molars, with RVCAL and RHCAL ≥ 3 mm were considered. Patients who had undergone periodontal therapy, smokers, pregnant and lactating women, teeth with Grade II mobility and patients under medications were excluded from the study.
The eligible samples were screened and randomly assigned by lottery method by investigator KRR into PRF (Group A) and GTR + Allograft (Group B). The treatment was performed by investigator SA who was blinded to the randomization process.
Clinical Parameters
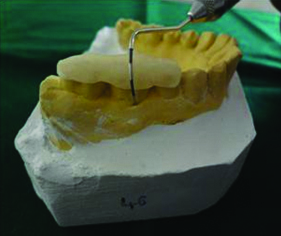
The clinical parameters were recorded at baseline and nine months (PI, PD, RVCAL, RHCAL, GML, and RVGBF). All the clinical measurements were standardized using acrylic stent with grooves, which were fabricated on the study model of the patients. The recordings were made using Nabers probe for RHCAL and UNC-15 probe for RVCAL and probing depths [Table/Fig-2].
Occlusal stent with Nabers probe for RHCAL measurement.
Vertical Clinical Attachment Level
The VCAL was calculated by measuring the distance from the reference point (RP) to the Base of the Pocket (BOP) and subtracting by the distance from the RP to the CEJ.
VCAL = (RP- BOP) – (RP- CEJ)
Horizontal Clinical Attachment Level
The HCAL was estimated from a fixed reference point into furcation using Nabers probe.
Gingival Marginal Level (GML)
GML was calculated by measuring the distance from Cemento-Enamel Junction (CEJ) to fixed Reference Point (RP) and subtracting it by the distance from the RP to Gingival Margin (GM) [5].
GML = (RP – CEJ) − (RP – GM)
Radiological Assessment
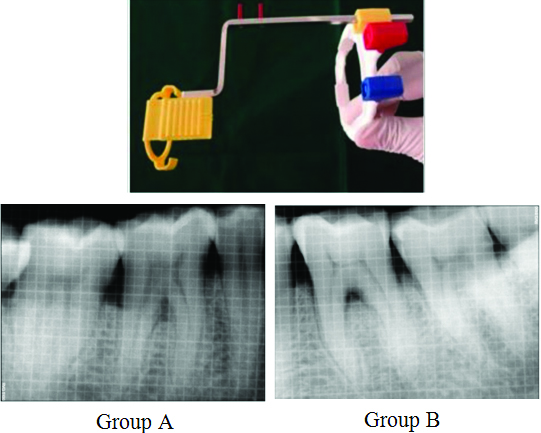
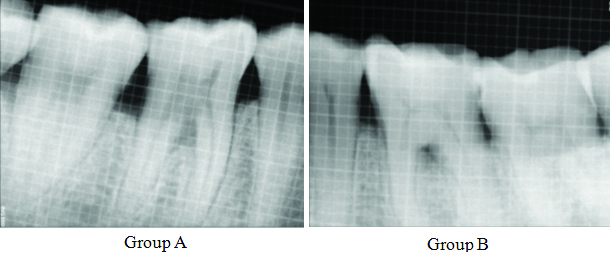
RVG images were taken preoperatively and nine months postoperatively. Long cone paralleling technique, a holding device and aiming rim were used to standardise the radiographic technique. SOPRO imaging software was used for measurements with the use of grids [5]. The measurements were recorded from the furcation fornix by calculating the radiolucency in the furcation area in millimetres ([Table/Fig-3], Group A, Group B).
RVG Sensor holder and aiming device, preoperative RVG using grid (Group A) and (Group B).
Pre-Surgical Procedure
All the patients, were given detailed instructions on selfperformed plaque control measures and were administered phase-I periodontal therapy.
Surgical Procedure
Eight weeks after Phase I therapy, the sites to undergo surgery were reassessed to ascertain their inclusion for the surgical procedure.
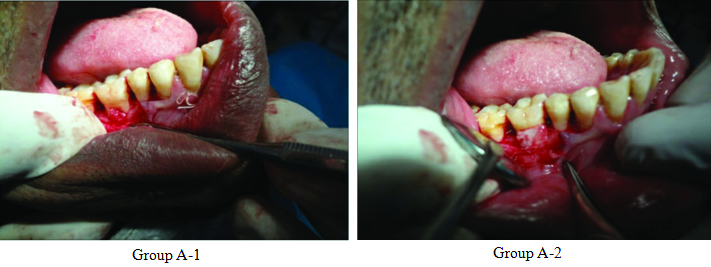
After anaesthetising the operative site using local anaesthesia, the crevicular and interdental incisions were made using no 15 bard parker blade. A full thickness mucoperiosteal flap was reflected and granulation tissue was debrided followed by thorough root planing using the furcation curettes and ultrasonic tips. Thorough irrigation was done with 0.9% normal saline and Group A sites were treated with PRF as a graft and as a membrane [Table/Fig-4],
PRF as a graft (Group A-1), PRF as a membrane (Group A-2).
Protocol for Platelet Rich Fibrin Preparation
Patients own blood from antecubital vein was drawn, transferred to sterile glass test tubes without any anticoagulants. These test tubes were centrifuged for 10 minutes at 3000 rpm in a centrifuge (Remi). After centrifugation, the clot obtained was compressed between sterile gauze piece and was used as a membrane and graft.
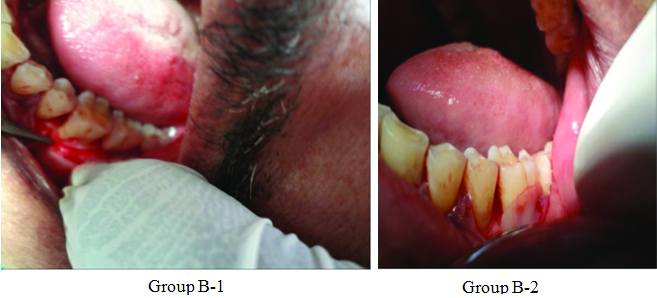
Group B sites were treated using combination of Demineralized Freeze Dried Bone Allograft (DFDB) 500-1040 microns from Tata Memorial Tissue Bank Mumbai, as a graft material and Healiguide (Advanced Biotech Products Pvt. Ltd., Tamilnadu) 20 mm x 30 mm as GTR membrane. The graft material was mixed with saline and used in increments and condensed well into the bone defect in the furcation area. The membrane was cut according to the defect size and placed 2 mm-3 mm apical to the defect so as to have a broad base and provide stability to the graft material and it was secured to the defect using a sling suture using 5-0 (Vicryl – Johnson and Johnson) absorbable suture material. Sutures were placed and COE-pack was given [Table/Fig-5].
Allograft placement (Group B-1), GTR placement before resizing (Group B-2).
Postoperative Care
All the patients received appropriate antibiotics (Amoxicillin 500 mg thrice daily for five days) and analgesics (Aceclofenac 100 mg and Paracetamol 325 mg thrice daily for four days) after which postoperative care was explained to them which included chlorhexidine rinses (0.12%) twice daily for two week period. At the end of one week, each patient was reassessed and instructed about proper oral hygiene measures, and examined once a week, up to one month after surgery, and again, at third and ninth months. Clinical parameters were reassessed after nine months using the prefabricated acrylic stent and bone fill was estimated using radio-visiography of the surgical site [Table/Fig-6].
RVG postoperative nine months follow up using grid (Group A) and (Group B).
Primary and Secondary Outcome Measures
The primary outcome measures examined were bone defect fill, RVCAL and RHCAL. The secondary outcomes included the measurement of the clinical parameters (PI, GML and PD).
Statistical Analysis
Based on a previous study SPSS version 14.0 was used and a p-value of less than 0.05 was considered to be statistically significant. Difference between the mean scores between baseline and follow up was assessed using paired t-test. Comparison of mean scores between the groups at each interval was done using independent sample t-test.
Results
In the present study, 22 defects in 22 patients, 14 male and eight females with age group of 30-50 years were examined. Patients were divided in two groups: Group A (n=11) and Group B (n=11).
Primary Outcome Measures
The RVCAL at baseline was 12.03±1.04 and at nine months post surgery was 8.42±0.97 in Group A, (p<0.001). The radiographic linear bone fill (RVGBF) at baseline was 13.0±0.89 in Group A and at nine months post surgery was 9.91±0.54 (p<0.001) [Table/Fig-7].
Intra-group comparison between Group A and Group B using Paired t-test.
| Group | Variables | Baseline | 9 months | Difference | p-value |
|---|
| Mean | SD | Mean | SD | Mean | SD |
|---|
| A | PI | 1.05 | .16 | .67 | .11 | 0.38 | 0.09 | <0.001 Sig |
| PD | 4.01 | .55 | 2.75 | .35 | 1.25 | 0.66 | <0.001 Sig |
| HCAL | 5.55 | .52 | 3.09 | .30 | 2.45 | 0.52 | <0.001 Sig |
| GML | 8.24 | 1.36 | 7.54 | 1.23 | 0.70 | 0.41 | <0.001 Sig |
| RVCAL | 12.03 | 1.04 | 8.42 | .97 | 3.61 | 0.78 | <0.001 Sig |
| RVGBF | 13.00 | .89 | 9.91 | .54 | 3.09 | 0.83 | <0.001 Sig |
| B | PI | 1.07 | .08 | .73 | .10 | 0.35 | 0.11 | <0.001 Sig |
| PD | 3.91 | .54 | 2.75 | .25 | 1.15 | 0.59 | <0.001 Sig |
| HCAL | 5.55 | .52 | 3.00 | .45 | 2.55 | 0.52 | <0.001 Sig |
| GML | 8.22 | .82 | 7.39 | .99 | 0.83 | 0.75 | 0.004 Sig |
| RVCAL | 11.69 | 1.29 | 7.50 | .99 | 4.19 | 0.99 | <0.001 Sig |
| RVGBF | 11.64 | 1.03 | 9.09 | .70 | 2.55 | 0.52 | <0.001 Sig |
*PI-Plaque Index, PD-Probing depth, HCAL-Horizontal clinical attachment level, GML-Gingival marginal level, RVCAL-Relative vertical clinical attachment level, RVGBF-Radiovisiograph Bone fill
†SD- Standard deviation
RVCAL at baseline was 11.69±1.29 in Group B and at nine months post surgery was 7.50±0.99 in Group B (p-value<0.001). RVGBF at baseline was11.64±1.03 and at nine months post surgery was 9.09±0.70 in Group B (p<0.001) which was considered statistically significant.
Intergroup comparison showed statistically significant difference of RVCAL (p-value=0.04 Sig) and RVGBF (p-value=0.006 Sig) in relation to PRF group (Group A) when compared to allograft plus GTR group (Group B), nine months post surgery [Table/Fig-8].
Intergroup comparison between Group A and Group B using independent sample t-test.
| Variables | Group | p-value |
|---|
| A (n=11) | B (n=11) |
|---|
| Mean | SD | Mean | SD |
|---|
| PI_B | 1.05 | .16 | 1.07 | .08 | 0.744; NS |
| PI_9 | .67 | .11 | .73 | .10 | 0.24; NS |
| PD_B | 4.01 | .55 | 3.91 | .54 | 0.67; NS |
| PD_9 | 2.75 | .35 | 2.75 | .25 | 1; NS |
| HCAL_B | 5.55 | .52 | 5.55 | .52 | 1; NS |
| HCAL_9 | 3.09 | .30 | 3.00 | .45 | 0.582; NS |
| GML_B | 8.24 | 1.36 | 8.22 | .82 | 0.97; NS |
| GML_9 | 7.54 | 1.23 | 7.39 | .99 | 0.763; NS |
| RVcal_B | 12.03 | 1.04 | 11.69 | 1.29 | 0.509; NS |
| RVcal_9 | 8.42 | .97 | 7.50 | .99 | 0.04; Sig |
| RVGBF_B | 13.00 | .89 | 11.64 | 1.03 | 0.003; Sig |
| RVGBF_9 | 9.91 | .54 | 9.09 | .70 | 0.006; Sig |
*PI-Plaque index, PD-Probing depth, HCAL-Horizontal clinical attachment level, GML-Gingival marginal level, RVCAL-Relative vertical clinical attachment level, RVGBF-Radiovisiograph Bone fill
† _B – At baseline; ‡ _9 – At 9 months; § -Standard deviation
Secondary outcome measures
There was a statistically significant difference in all the clinical parameters from baseline to nine months within the group [Table/Fig-7], however when intergroup comparison was done the results were not statistically significant [Table/Fig-8].
Discussion
Periodontitis is a chronic inflammatory disease, with gingival inflammation, pocket formation and associated loss of alveolar bone. Among various treatment modalities, periodontal regeneration which is the most challenging has come to the forefront of periodontal research and practice. A number of techniques using autogenous, allogenic, xenogenic and alloplastic bone graft materials for regeneration purposes have been reported, with limited success [6,7].
In a study evaluating bioabsorbable barrier membranes with and without DFDBA in human molar Grade II furcation defects, the researchers observed that the probing depth decreased postoperatively in the group administered GTR with DFDBA [8].
In a study assessing the effects of bioresorbable and nonresorbable GTR membranes in Grade II furcation defects the authors concluded that both barrier membranes increased the clinical attachment level postoperatively, which was maintained for 10 years in 83% of the defects. However, they did not find a difference between the two types of barrier membranes used [9].
A study used DFDBA for mandibular Grade II furcation defects. It was observed that composite autogenous grafts and DFDBA showed more than 50% bone fill at re-entry in 10 out of the 13 furcation defects. Moreover, four out of the 13 defects demonstrated complete bone fill [10].
In another long term evaluation of GTR treatment of furcation defects the researchers found that only 51% of mandibular Grade II furcation defects treated with membrane has responded with complete furcation fill at the short term evaluation and maintained their integrity five years later. Of the Grade II furcation defects originally treated with membrane and DFDBA, 74% gave total bone fill at both the evaluations [11].
In this study also, there was a significant improvement in RVCAL (12.03±8.42), HCAL (5.55±3.09) and RVG bone fill (13.00±9.91) from baseline to nine months follow up when DFDBA and GTR was used.
The success of GTR and allograft in the treatment of Grade II furcations has already been proved, but a comparison between GTR+ Allograft and PRF (graft & membrane) as treatment modalities for Grade II furcation involvement has not been done, hence, this study aimed to find out the better of the two pertaining to their performance.
Growth factors have been studied and used a lot these days due to their superior results in wound healing. Many growth factors like Transforming Growth Factor β (TGF β), Insulin Like Growth Factor (IGF), Fibroblast Growth Factor (FGF) have been researched on, and Platelet Derived Growth Factor (PDGF) also belongs to the same family. PDGF are biologic mediators which regulate the proliferation, differentiation and chemotaxis of cells [3].
Studies related to Platelet Rich Plasma (PRP) have been done, but extensive use of PRP has been deferred because of laborious preparation time, and cross reactivity with bovine thrombin which has been used as an anticoagulant.
Choukron J et al., in France introduced PRF which belongs to the second generation of platelet concentrates [12]. In the dental field, it was initially used in oral and maxillofacial surgery and later on in implantology. Periodontal literature provides promising results following application of PRF combined with other graft materials in furcation defects [13]. However, the regenerative potential of PRF alone in furcation defects was assessed only in few studies.
PRF has a significant role to play in tissue remodelling due to its inherent capability of slow release of growth factors from one week to 28 days post insertion. No thrombin is added to PRF which makes it more stable as a natural fibrin meshwork preventing proteolysis of growth factors [14].
In their study employing PRF in furcations the authors observed that there was a greater reduction in probing depths when compared to the control sites, treated by open flap debridement alone, nine months postoperatively. The difference observed was 2.17 mm between the groups. Moreover, the test sites presented with a significantly greater vertical defect fill (50.8±6.24) than the control sites (16.7±6.42) [13].
In this study also, there was a significant improvement in clinical parameters like PD, HCAL, VCAL, GML and linear bone fill.
Wu CL et al., attempted to elucidate the effects of PRF on human osteoblasts. They observed that PRF alone stimulated cell attachment compared with untreated controls [15]. PRF has been observed to increase the proliferation and attachment of osteoblasts and also increase the production of proteins of the collagen lineage, thus, facilitating bone regeneration. PRF was applied as a sole filling material in periodontal defects. They observed a reduction in PD and a gain in clinical attachment level; with osseous defect fill radiologically [16]
While measuring all the soft tissue parameters, a fixed reference point was made using acrylic stent in this study. The authors have reported that measurements using a stent appear to be better than measurements made using CEJ [17].
Many studies have been done using PRF as a sole regenerative material either in intrabony defects or furcation defects [Table/Fig-9] [13,18-21], however, as per our knowledge very few studies have been done using PRF both as a graft and a membrane [13]. Thus, in this study the reparative and regenerative functions of PRF in a dual role (graft and membrane) was assessed.
Clinical studies using PRP/PRF [13,18-21].
| Clinical study | Characteristics of the defect | Intervention/Test | Comparison/Control | Clinical and radiographic parameters | Follow up in months | Results |
|---|
| Sharma A and Pradeep AR [13] | Grade II furcation defects in Mandibular molars with PD≥5 mm and horizontal PD≥3 mm | PRF as a graft and a membrane | OFD | PI, SBI, PD, GML, RHCAL, RVCAL, Linear bone fill | 9 Months | 9 months postoperatively, test sites presented with a significantly greater reduction in PD than control sites, with a difference of 2.17 mm. Test sites also presented with a significantly greater vertical defect fill (50.8 ±6.24) than the control sites (16.7 ± 6.42) at nine months |
| Thorat MK et al., [18] | Interproximal IBD≥3 mm along with interproximal PD≥5 mm | PRF as a graft | OFD | PD, CAL, GMP, IBD, bone defect fill | 9 Months | Mean PD reduction(4.56±0.37>3.56± 0.27) and CAL gain (3.69±0.44 > 2.13±0.43) in test group was found to be more compared with that of control group. |
| Rosamma Joseph V et al., [19] | Contralateral interproximal IBD with probing pocket depth PD≥6 mm, CAL≥5 mm and IBD≥4 mm | PRF as a graft | OFD | PD, CAL, IBD, Recession | 12 Months | Postoperative differences observed between the two groups were 2.27±0.29 mm (p<0.001) for probing pocket depth, 3.33±0.35 mm (p<0.001) for clinical attachment level and 1.29±0.32 mm (p<0.001) for radiographic infrabony defect depth reduction, all in favor of the experimental group. |
| Pradeep AR et al., [20] | 3 wall IBD≥3 mm with interproximal PD≥5 mm | PRF as a graft | PRP, OFD | PD, CAL, GMP IBD, Bone defect fill | 9 Months | Mean PD reduction and CAL gain were greater in PRF (3.77 ± 1.19 mm and 3.17 ± 1.29 mm) and PRP (3.77 ± 1.07 mm and 2.93 ± 1.08 mm) groups than the control group (2.97 ± 0.93 mm and 2.83 ± 0.91 mm). Furthermore, significantly greater percentage of mean bone fill was found in the PRF (55.41% ± 11.39%) and PRP (56.85% ± 14.01%) groups compared with the control (1.56% ± 15.12%) group. |
| Bajaj P, et al., [21] | Grade II furcation defects in Mandibular molars | PRF and PRP as grafts | OFD | PD, RVCAL, RHCAL, GML | 9 Months | RVCAL gain was greater in PRF (2.87±0.85 mm) and PRP (2.71±1.04 mm) sites as compared to control site (1.37±0.58 mm), and RHCAL gain was statistically significantly greater in both PRF and PRP than in the control group. |
*PD-Probing depth, PI-Plaque Index, SBI-Sulcus bleeding index, GML-Gingival marginal level, GMP-Gingival marginal position, CAL- Clinical attachment level, RVCAL-Relative vertical clinical attachment level, RHCAL- Relative horizontal clinical attachment level, IBD-Intrabony defect.
† PRF- Platelet rich fibrin; ‡ PRP-Platelet rich plasma; § OFD-Open flap debridement; Clinical studies using PRP/PRF
In this study, there was a reduction of all the clinical parameters post surgically. The improvement in clinical parameters was in agreement with earlier reports by Pradeep AR et al., [20]. However, their study compared sites treated with PRF to those with Open flap debridement, whereas, this study differed in that it compared the efficacy of PRF as a graft as well as a membrane (in the treatment of mandibular molar Grade II furcation defects) to allograft and Healiguide. The PRF group has shown a significant improvement in all the parameters.
Pratap S et al., in their study used radio-visiography technique as an additional method to observe the possible changes in the mandibular molar Grade II furcation region preoperatively and six months postoperatively [5].
In this study also, radio-visio-graphy with grid using SOPRO software was applied to facilitate the measurement of bone fill post surgically.
In this study, it was observed that both PRF and GTR+ Allograft showed improvement in clinical parameters, though the improvement in RVCAL and RVGBF was better in the PRF group. In future, the dual role of PRF as graft and a membrane has to be proved by conducting many more clinical studies.
Limitation
This study did not follow a split mouth design. The follow up period in this study was nine months only. The beneficial effects of PRF would have been better substantiated, if the patients could have been followed up, on a long term basis (i.e.,) after 12-18 months.
Conclusion
Furcation involvement of posterior teeth is still a difficult area to treat. Platelet rich fibrin is one among the many regenerative materials which has shown promising results. This study showed a significant improvement in RVCAL and RVGBF in the PRF group when compared to allograft with GTR group. Thus PRF could be a better treatment option in the treatment of mandibular molar Grade II furcation defects. However many more long term studies with a larger sample size are required to gain insight into the beneficial effects of PRF as a regenerative material.
*PD-Probing depth, PI-Plaque Index, SBI-Sulcus bleeding index, GML-Gingival marginal level, GMP-Gingival marginal position, CAL- Clinical attachment level, RVCAL-Relative vertical clinical attachment level, RHCAL- Relative horizontal clinical attachment level, IBD-Intrabony defect.
† PRF- Platelet rich fibrin; ‡ PRP-Platelet rich plasma; § OFD-Open flap debridement; Clinical studies using PRP/PRF