The use of intracanal medications to disinfect the root canal system has been widely advocated as they eliminate bacteria in the root canal, prevent proliferation of micro-organisms between the appointments and also act as a physiochemical barrier, preventing root canal reinfection and nutrient supply to the vestigial bacteria [1]. Calcium hydroxide has commonly been the intracanal medicament of choice because of its wide range of action period of one to two months [2], but its depth of penetration into dentinal tubules is still unestimated [3,4]. Various conditions like persistent intraradicular and extraradicular infection by bacteria of species like Actinomyces israelii and Propionibacterium propionicum, foreign body reactions, presence of cyst especially those containing cholesterol crystals and fibrous scar tissue healing towards conventional treatment are associated with endodontic failures [5]. Of all these factors, the main culprit for the root canal treatment failure is the persistence of micro-organisms in the apical part of the root filled teeth. The species Enterococcus faecalis, and fungi have shown to be resistant to the effects of commonly used intracanal medicament, calcium hydroxide, and have been recovered in high proportions form approximately one third of the canals of root with endodontic failures [4-9]. Chlorhexidine has shown marked efficacy against Enterococcus faecalis and Candida spp. and is also known to bind to dental hard tissues having certain residual effect [4,10]. Thus, it has been recommended as a viable interappointment dressing, provided that an adequate delivery system is used [11]. Various challenges which have to be dealt with are: excessive or premature peaking of the chlorhexidine leading to possible side effects or futility like any other intracanal medicament. Therefore, this experimental bioengineering project was undertaken to develop, pharmacologically characterize and microbiologically analyse a local drug delivery system in injectable Solution (Sol) form, which can transform in a gel on exposure to acidic conditions thereby simulating an infected canal. The proposed formulation will thereby release chlorhexidine into the root canal in a sustained and controlled manner. This work is a continued attempt to develop various intracanal sustained release devices containing chlorhexidine by various researchers [2,11].
Materials and Methods
The following in vitro study was carried out in JSS Dental College, Mysuru after obtaining the Institute’s Ethical Clearance from February 2005 to March 2006.
The following materials, accessories and equipments were employed in the present project to formulate and evaluate the sustained pH dependent drug delivery system: Chlorhexidine digluconate (Sigma Aldrich Company, USA), GELRITE® Gellan gum (G1910-250G, Sigma Aldrich Company, USA), dialysis membrane-50 (LA-387, Hi Media, India), viscometer (Brookfield, USA), UV visible spectroscopy (Shimadzu. UV-1601, Japan), freeze drier, Fourier Transform Infrared (FTIR) spectrometer (Perkin Elmer System 2000).
Preparation of the Formulations
GELRITE® Gellan gum (0.1 and 0.2%) (polymer) was dissolved in hot double distilled water for injection at 70°C under laminar flow, by continuous stirring at 40°C and filtered to remove particulate matter using muslin cloth (pore size: 75 μm). The quantity of chlorhexidine drug (0.1% and 0.2%) was added to the polymeric solution to give final concentration and stirred until both solutions were completely miscible. Two formulations without drug as a control (F1,F2) and four test formulations (F3-F6) containing the drug were developed and filled in 10 ml amber colored glass vials, capped with rubber bungs and sealed with aluminum caps. Formulations F3 and F5 were prepared by adding 0.05 ml of 20% w/v of chlorhexidine digluconate aqueous solution to 9.95 ml of 0.1% and 0.2 % w/v GELRITE® solution respectively. Similarly, formulations F4 and F6 were prepared by adding 0.1 ml of 20% w/v of chlorhexidine digluconate aqueous solution to 9.9 ml of 0.1% and 0.2% w/v GELRITE® solution respectively. In the final pack, the formulations were terminally sterilized by autoclaving at 121°C at 15 Pa for 20 minutes. Sterilized formulations were stored at room temperature until use.
Pharmacological Evaluation of the Formulations
Drug Content Uniformity (DCU): The preparations were shaken for two to three minutes manually and 100 microlitre of the preparation was transferred aseptically to sterile 25 ml volumetric flask with micropipette and final volume was made up with distilled water. The chlorhexidine digluconate concentration was determined at 254 nm using UV visible spectroscopy (Shimadzu. UV-1601, Japan) after suitable dilution.
Gelation studies: Gelation studies were done in Teflon Gelation cells, which were a kind of cylindrical reservoir sufficient to hold 3 ml of gelation solution (relevant pH adjustments done with 0.1 N HCl/0.1 N NaOH solutions). A 250 μl transparent plastic cups were placed within these cells to hold the formed gel samples. A 2 ml of gelation solution was gradually added to the 100 μl preparation (previously placed into the cup using micropipette). The preparation was examined visually for the gel formation.
Rheological studies: Viscosity measurement of the conceived formulation was performed out on a cone (0.8 degree) and plate geometric viscometer (Brookfield, USA) using spindle CP 40. Viscosity of sample solutions was checked out at varied angular velocities, but at a temperature of 37±1°C. A particular cycle comprised of change of angular velocity from 0.5 to 100 rpm at a controlled ramp speed. Post time period of six seconds at 0.5 rpm, the velocity was hiked to 100 rpm with the congruous wait at each speed. The order of angular velocity was reversed (100 rpm to 0.5 rpm) with analogous wait of six seconds. The viscosity was calculated by using average of two readings. These evaluations were performed in triplicate.
In vitro drug release studies: A presoaked dialysis membrane- 50, having average flat width 24.26 mm, average diameter of 14.3 mm and capacity of 1.61 ml/cm (approximately) was utilized for diffusion. The hydrated membrane was used for diffusion study. A 2 ml of sol-gel was kept in a dialysis membrane, which was sealed on both sides. The dialysis tube was then placed in amber colored glass bottle containing 50 ml of distilled water. The release studies were performed at 37±1°C. A 5 ml of recipient solution was withdrawn at specified intervals initially and at suitable intervals later and replaced with an equal amount of fresh distilled water. Samples were analysed for drug content at 254 nm by UV visible spectroscopy (Shimadzu. UV-1601, Japan).
Drug excipient compatible study: FTIR spectroscopy was employed using FTIR spectrometer (PerkinElmer System 2000) to ascertain the compatibility of drug with the excipient (polymer). The individual drug, excipient and the drug with excipient were separately scanned. All the spectra were compared for common peaks.
Microbiological Evaluation of the Formulations
All the cultures used in the study were activated by first inoculating isolated colonies to freshly sterilized Brain Heart Infusion (BHI) broth prepared from dehydrated media (Hi Media, India). The tubes were incubated at 37°C for 18 hours and subcultured to fresh BHI broth to obtain an 18 hour young culture for antimicrobial susceptibility studies. Cultures used in the present study were Enterococcus faecalis (freshly isolated in the laboratory) and Candida albicans (ATCC® 14053™).
Disc and well diffusion studies: Three media, Tryptone Soya agar, BHI agar and Mueller Hinton agar (HiMedia, India) were used for this study. Antimicrobial discs for the formulations were prepared from Whatman filter paper no.1 by punching discs of 6.5 mm diameter and wrapping them in an aluminum foil, prior to sterilizing the same in hot air oven at 140°C for 30 minutes. A total of hundred dry discs were prepared by impregnating each disc with 10 μl of formulation with the help of a micropipette such that the final strength of each disc was 10 μg for F3 and F5 formulation (0.1% drug concentration) and 20 μg for F4 and F6 formulation (0.2% drug concentration). Each young broth culture was poured on media and allowed to remain for two minutes. The broth was then discarded and the medium surface was air dried in a laminar flow cabinet for 10 minutes. The antimicrobial discs were then placed on the medium surface and the plates were incubated at 37°C for 18-24 hour. The zone of inhibition in mm was recorded. Similarly zone of inhibition was measured with well diffusion technique, in which wells of 5 mm diameter and approximately 4 mm depth were punched into agar plates with sterile cork borer. The well was loaded with the measured quantity (10 ml) of the test formulations. Zones of inhibition were evaluated.
MIC: This was carried out in BHI broth (HiMedia pH 7.4±0.2). The medium was prepared and distributed in 5 ml volume in each tube and autoclaved at 110°C for ten minutes. The various formulations were added to the tubes in the volumes mentioned. For the evaluation of MIC, 0.05 ml of young BHI broth culture of E. faecalis and C. albicans ATCC® 14053™ was used by inoculating them to each tube containing different strength of the formulations. The tubes were incubated at 37°C for 18 hours and turbidity was recorded to indicate growth or otherwise.
MIC was calculated per ml by observing these tubes for growth. The first tube in any set that demonstrated no growth was taken into account for the calculation.
Results
Results of Pharmacological Evaluation
Drug content uniformity: The drug concentration was determined at 254 nm (Shimadzu. UV-1601, Japan) after suitable dilution. All the formulations showed high level of drug content uniformity thereby indicating high degree of miscibility [Table/Fig-1].
Prepared formulations and pharmacological characterization.
| FormulationCode (F) | Polymer Concentration (GELRITE Gellan Gum)(%) | Drug Concentration (Chlorhexidine)(%) | DCU (%) Percentage of Drug Present in Formulation | Gelling Capacity |
|---|
| F1 | 0.1% | nil | nil | nil |
| F2 | 0.2% | nil | nil | nil |
| F3 | 0.1% | 0.1% | 99.18±0.48 | ++ |
| F4 | 0.1% | 0.2% | 99.09±0.51 | ++ |
| F5 | 0.2% | 0.1% | 99.64±0.86 | +++ |
| F6 | 0.2% | 0.2% | 99.43±0.68 | +++ |
| | | M±SD, N=6 | ++ Less Stiff Gel+++ Firm Stiff Gel |
Gelation studies: Formulation with 0.1% concentration of polymer i.e., F3 and F4 formed less stiff gel immediately whereas formulation with 0.2% concentration of polymer i.e., F5 and F6 formed firm stiff gels immediately [Table/Fig-1].
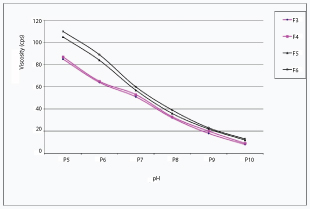
Rheological studies: All the test formulations showed inverse relation with the angular velocity in the viscometer. Formulations containing 0.2% polymer (F5 and F6) demonstrated higher viscosity than formulations containing 0.1% polymer (F3 and F4) at all the speeds. With the increase in pH, all the test formulations showed decrease in viscosity at a constant angular velocity of 20 rpm. At a higher pH (pH=10) the formulations were present in Sol form and showed low viscosity whereas the same solutions converted into gel form at lower pH and demonstrated higher viscosity [Table/Fig-2].
Graph showing rheological studies of various formulations.
In vitro drug release studies: In the solution form the drug was available in the recipient solution within one hour. The formulations containing 0.1% polymer (F3 and F4) showed close to 80% release within the first two hours of the experiment. Both the solutions showed similar release profile when plotted against time. Formulations containing 0.2% polymer (F5 and F6) demonstrated 80% release of drug at close to four hours instead. Both the formulations showed similar release profile when plotted against time. Close to 100% of the drug was released at the end of six hours. On the contrary the dissolution profile of drug from gel form showed a delayed release of drug in gel form. All the test preparations showed similar drug release profile. Close to only 50 to 75% of the drug from the gel form was observed to be released at the end of 96 hours in contrast to drug release from solution form where close to 100 % drug was available in recipient solution at the end of six hours [Table/Fig-3].
Dissolution profile of drug in Sol and gel form.
| Time (hrs.) | Cumulative percent release of Chlorhexidine from Sol form | Cumulative percent release of Chlorhexidine from Gel form |
|---|
| F3 | F4 | F5 | F6 | F3 | F4 | F5 | F6 |
|---|
| 1 | 39.46 | 42.58 | 28.72 | 30.17 | 3.18 | 5.39 | 2.24 | 3.25 |
| 2 | 80.25 | 83.46 | 45.29 | 49.45 | 4.02 | 7.16 | 3.26 | 3.85 |
| 4 | 94.43 | 95.28 | 80.83 | 85.38 | 5.65 | 9.97 | 4.35 | 5.65 |
| 6 | 97.23 | 99.25 | 93.24 | 95.64 | 8.51 | 11.09 | 6.25 | 7.14 |
| 8 | - | - | - | - | 12.7 | 14.07 | 9.12 | 10.79 |
| 12 | - | - | - | - | 20 | 22.12 | 12.25 | 14.55 |
| 24 | - | - | - | - | 27.43 | 32.25 | 19.85 | 20.65 |
| 48 | - | - | - | - | 42.76 | 48.5 | 32.56 | 23.55 |
| 72 | - | - | - | - | 61.22 | 66.78 | 44.78 | 35.28 |
| 96 | - | - | - | - | 71.68 | 77.29 | 61.57 | 50.25 |
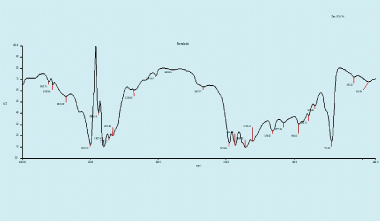
Drug excipient (polymer) compatible result: The individual drug, excipient and the drug with excipient were separately scanned. All the spectra were compared for common peaks. All the spectra obtained in the FTIR were compared for common peaks revealed, and the drug and the excipient maintained their individual characteristics and demonstrated compatibility [Table/Fig-4].
FTIR spectroscopy for formulation (Drug-Chlorohexidine with Excipient GELRITE Gellan Gum-polymer).
Results of Microbiological Evaluation
Disc and well diffusion technique: All the formulations containing drug showed good efficacy against the test micro-organisms in both disc diffusion method and well diffusion method. Tryptone Soya agar medium did not support the growth of Enterococcus faecalis [Table/Fig-5,6 and 7].
Petridish showing zones of inhibition.
Disc diffusion technique-Zone of iinhibition(mm) (Disc diameter-6.5 mm, Vol in each disc-10 μl).
| Formulation | Tryptone Soya agar | Brain Heart Infusion agar | Mueller Hinton agar |
|---|
| C.albicansATCC® 14053™ | E. faecalis | C.albicansATCC® 14053™ | E. faecalis | C.albicansATCC® 14053™ | E. faecalis |
|---|
| F-1 | -- | -- | -- | -- | -- | -- |
| F-2 | -- | -- | -- | -- | -- | -- |
| F-3 | 8mm | -- | 9mm | 7mm | 10mm | 10mm |
| F-4 | 10mm | -- | 10mm | 9mm | 13mm | 12mm |
| F-5 | 6mm | -- | 7mm | 7mm | 9mm | 7mm |
| F-6 | 9mm | -- | 10mm | 8mm | 13mm | 10mm |
Cultures used: Candida albicans ATCC® 14053™ and E. faecalis. Three media used: Tryptone Soya agar, BHI agar, Mueller Hinton agar
Well diffusion technique-Zone of inhibition (mm) (Volume in each well 10μl).
| Formulation | Tryptone Soya agar | Brain Heart Infusion agar | Mueller Hinton agar |
|---|
| C. albicansATCC® 14053™ | E. faecalis | C. albicansATCC® 14053™ | E. faecalis | C. albicansATCC® 14053™ | E. faecalis |
|---|
| F-1 | -- | -- | -- | -- | -- | -- |
| F-2 | -- | -- | -- | -- | -- | -- |
| F-3 | 7 mm | -- | 10 mm | 8 mm | 11mm | 10 mm |
| F-4 | 9 mm | -- | 11mm | 11 mm | 12 mm | 12 mm |
| F-5 | 6 mm | -- | 8 mm | 8 mm | 10 mm | 8 mm |
| F-6 | 11mm | -- | 12 mm | 10 mm | 12 mm | 11mm |
MIC: MIC values against Candida albicans (ATCC® 14053™) was observed as 4 μl/ml of both F3 and F5 formulation [Table/Fig-8] Whereas MIC values against Enterococcus faecalis was observed to be 3.2 μl/ml and 6 μl/ml of F3 and F5 formulation respectively [Table/Fig-9]. Since formulations F4 and F6 had higher concentration of drug, they were not included in MIC evaluation.
MIC of different formulations to C. albicans (ATCC® 14053™ culture).
| FORMULATION | Amount Of Drug (μL)/5mL medium |
|---|
| 1 | 5 | 7.5 | 10 | 20 | 30 | 40 |
|---|
| F1 | + | + | + | + | + | + | + |
| F2 | + | + | + | + | + | + | + |
| F3 | + | + | + | + | - | - | - |
| F5 | + | + | + | + | - | - | - |
F-1 => Polymer 0.1%, F-2 => Polymer 0.2%, F-3 => Polymer 0.1% + Drug 0.1%, F-5 => Polymer 0.2% + Drug 0.1%, +=>Growth -=>No growth
MIC of different formulations to Enterococcus faecalis.
| Formulation | Amount Of Drug (μl)/5ml medium |
|---|
| 12 | 14 | 16 | 18 | 20 | 30 |
|---|
| F-1 | + | + | + | + | + | + |
| F-2 | + | + | + | + | + | + |
| F-3 | + | + | - | - | - | - |
| F-4 | - | - | - | - | - | - |
| F-5 | + | + | + | + | + | - |
| F-6 | - | - | - | - | - | - |
MIC : 3.2μl/ml of F–3 formulation and 6 μl/ml of F-5 formulation
Note: F4 and F6 have higher drug concentration than F3 and F5 and were therefore inhibitory even at the lowest concentration.
*F-1 => Polymer 0.1%, F-2 => Polymer 0.2%, F-3 => Polymer 0.1% + Drug 0.1%, F-4 => Polymer 0.1% + Drug 0.2%, F-5 => Polymer 0.2% + Drug 0.1%, F-6 => Polymer 0.2% + Drug 0.2%,
+=>Growth
-=>No growth
Discussion
Enterococcus faecalis and Candida albicans have been commonly associated with failed endodontic therapy. In most instances, the incidence of failure has been correlated by selective growth of these organisms. The presence of abscess in periapical areas of a tooth resistant to endodontic therapy is a common feature and often presents an acidic environment with reduced pH approximately close to six [12]. In the present study, a local drug delivery system was experimentally developed to release chlorhexidine into the root canal in sustained and controlled manner, as a novel method of suppressing growth of the above mentioned organisms and reducing the rate of failures of the endodontic therapy. The presence of the acidic conditions trigger the conversion of the drug in sol into the gel form and release the drug to fight persistent infections in a sustained manner. This method of drug delivery could possibly eliminate the need of use of systemic antibiotics. The controlled release system maintains a fairly constant concentration of the drug for extended period of time. Excessive or premature peaking of the drug is avoided and side effects may be reduced. In addition with the use of such formulations, the dosage frequently is reduced and the therapy is simplified. This work is a continued attempt to develop various intracanal sustained release devices containing chlorhexidine by various researchers [2,11]. The clear advantage is in delivering antimicrobial effects directly to the targeted site, without the need of administering systemic antibiotics.
The study was divided into three categories, formulation of the proposed drug delivery system, its pharmaceutical evaluation followed by microbiological evaluation of the formulations.
The viscous bases used in many irrigants are little soluble in water, leaving behind residues on dentin wall that damage the final obturation of the root canal system. Therefore the gel base used in the present study was a Hydrogel (GELRITE®) that is a non ionic, widely used in food industry for gelling purposes [13]. GELRITE® is a deacetylated Gellan gum. It has a high molecular mass and is ion activated in situ gel i.e., pH responsive. Chemically it is linear anionic hetropolysaccharide. Gellan gum is biodegradable and approved by FDA for usage in food industry. It is categorized as Generally Regarded as Safe (GRAS). Hydrogels on a whole are three dimensional, hydrophilic, polymeric networks capable of imbibing large amounts of water or biological fluids. They are insoluble due to the presence of chemical cross links, such as entanglement or crystallites. High water content contributes to the biocompatibility if the material. Such systems are capable of adjusting drug release rates in response to a physiological need and are gaining rapid popularity [14]. With a single dose, desired therapeutic range of the drug can be maintained by the controlled drug release devices. These devices improve the patient compliance by rendering and localizing the drug to a particular compartment [15].
Localized delivery of the drug to a particular body compartment, which lowers the systemic drug level, reduces the need for follow up care, preserves medications that are rapidly destroyed by the body and increases patient comfort and/or improves compliance [15].
Chlorhexidine has been shown to be effective aid in preventing colonization of the surfaces of teeth and in removing dental plaque. Significant benefits to wound healing following endodontic, periodontal and oral surgical operations have been reported with the application of chlorhexidine [2,10,16-18]. Chlorhexidine is odorless, bitter tasting, white crystalline powder. Chemically chlorhexidine is a 1,6-Bis {N’-(p-chlorphenyl)-N5-biguanido} with the empirical formula C22H30C12N10. Its molecular weight is 505.48 and falls into the functional category of antimicrobial, preservative and an antiseptic. It has been shown in various studies that chlorhexidine is significantly more effective than calcium hydroxide in eliminating E. faecalis and Candida albicans especially in gel form [19].
The pharmaceutical evaluation of the formulations containing Chlorhexidine showed compatibility between the excipient (GELRITE® Gellan gum) and the drug (chlorhexidine digluconate). The high degree of DCU from the drawn samples indicated the same. Both the concentrations of polymer i.e., 0.1% and 0.2% showed good handling characteristics and were in solution form at room temperature. They can be easily carried in the syringe and injected into the canal as desired in a clinical situation. The drop in pH resulted in conversion of the polymer in solution form to the gel form in vitro. The similar change in pH is anticipated in the root canal infected with bacteria. With the increase in pH, all the test formulations showed decrease in viscosity at a constant angular velocity of 20 rpm. At a higher pH the formulations were present in sol form and showed low viscosity whereas the same solutions converted into gel form at lower pH and demonstrated higher viscosity.
Comparison of the drug release profile from the solution and gel form clearly indicated the potential use of the formulation in prolonging the availability of drug in therapeutic concentrations for extended period of time. Premature peak of the drug availability is avoided along with the potential side effects of high concentration of drugs. Secondly, the denaturing effect of the dentin and other products like endotoxins form bacterial metabolisms, limit the effectiveness of many drugs including calcium hydroxide [20]. With the gelling of the formulations it is presumed that the drug is fortified and prevented from the denaturing effect of the environment present in the canal.
All the formulations containing drug showed good efficacy against the test micro-organisms in both disc diffusion method and well diffusion method. Tryptone Soya agar medium did not support the growth of Enterococcus faecalis though. Chlorhexidine contained in various formulations resulted in an inhibition of the growth of the test organisms on the media plates. The diameter of the zones of inhibition was observed of the similar range. Chlorhexidine released from the experimental formulations had clear inhibitory effect on the test organisms. This observation favors the potential use of such a formulation as a medicament and interappointment dressing especially when an open canal or a persistent infection is encountered. The observed value of the MIC against the test organisms indicates the impending therapeutic value of the test formulations, which contained concentrations of chlorhexidine in extremely large amount. However, in some research, triple antibiotic paste was more effective than chlorhexidine followed by nano silver and normal saline [21].
Endodontics has evolved from a long history of embracing intracanal medicament to rejecting them in the 1970’s. The value of all historically used delivery vehicles, techniques and medications haven been re-evaluated. When an efficient delivery vehicle can be used in combination with an effective antimicrobial, the “intracanal medication pendulum” might swing back in favor of the use [22,23]. In our study, the gel containing chlorhexidine inhibited the growth of pathogens on media plates. Taken together, these experiments demonstrate in principle that there is merit in further exploring the potential of sol-gel containing chlorhexidine as interappointment medication in infected root canals.
Limitation
In a skeptical view of the foretasted research work, it can be said that the study could be extrapolated to compare chlorhexidine to other intracanal medicaments and inhibitory actions of the same medicament against other virulent micro-organisms could have been performed.
Conclusion
Within the scope of the above mentioned experiment, chlorhexidine is effective against Enterococcus faecalis and Candida albicans in vitro and the formulation has potential to increase biological half life of drug within the canal, thereby increasing its efficacy and reducing the possible side effects. The sol-gel drug delivery system can have potential use as a sustained release device for intracanal drug delivery. The study has proven the efficacy of sol-gel drug delivery system in ex vivo environment. Nevertheless its activity in animal studies and in vivo clinical trials is imperative before establishing it as an able drug delivery system.