Recent years have witnessed increasing interest in postoperative pain management by inhibiting trauma-induced nociceptive impulses and providing subjective comfort to the patient. Adequate analgesia in the postoperative period subsequently leads to early restoration of functions especially after orthopaedic surgery, as the degree of postoperative pain is closely related to arthrofibrosis as a result of diminished joint movements [1].
The axillary approach to the brachial plexus is the most popular because of its ease, reliability and safety [2], and its association with a very low complication rate as compared to other approaches [3] in the forearm surgeries.
Modern local anaesthetics are sufficiently effective and safe for use in regional nerve blocks, but the search for agents with lower incidences of systemic toxicity, lesser degree of motor blockade and longer duration of action continues. Brachial plexus blocks employing long acting local anaesthetics [4] have been tried in the past so as to increase duration of postoperative analgesia, but are associated with prolonged motor blockade leading to delayed restoration of limb movements in the postoperative period.
Thereby, various novel analgesic adjuvant to brachial plexus block including buprenorphine [5], dexamethasone [6], magnesium [7], and midazolam [8], the goal of which is to reduce the onset time, prolong the analgesic effect without the disadvantage of systemic side effects or prolonged motor blockade and allow for the reduction in the total dose of local anaesthetic, are being under trial. Recently, Alpha(α)-2-Adrenergic Receptor (AR) agonists have been the focus of interest by virtue of their excellent sedative, analgesic, anaesthetic sparing and haemodynamic stabilizing properties. Dexmedetomidine with a relatively high ratio of α2:α1 activity (1620:1 as compared to 220:1 for clonidine) is currently the most potent α2-AR agonist available. Few clinical studies have evaluated the effect of adding dexmedetomidine to local anaesthetics in the axillary block [9,10]. Till date only single study [11] has evaluated the dose response of dexmedetomidine as an adjuvant to 0.5% lignocaine in Intravenous Regional Anaesthesia (IVRA) and concluded that the addition of 1 μg/kg dexmedetomidine to lignocaine for IVRA showed significantly better improvement in the quality of anaesthesia and postoperative analgesia in comparison to 5 μg/kg dexmedetomidine.
We, therefore, intended to study the efficacy and safety of dexmedetomidine in two different doses as an adjuvant to lignocaine in forearm and hand surgeries under axillary brachial plexus block via transarterial approach, having most beneficial effect on duration of postoperative analgesia and thereby to find out the near ideal dose of dexmedetomidine as an adjuvant.
Materials and Methods
This study was carried out on 103 American Society of Anesthesiologist I/II patients of either gender, in the age group of 20-60 years, having fractures of forearm bones scheduled for open reduction and internal fixation under axillary brachial plexus block over a period of 24 months from December 2011 to December 2013.
Exclusion criteria included patient’s refusal for block, bleeding disorders, history of seizures, respiratory or cardiac diseases, partial block where supplementary anaesthesia was required and local infection at the site where needle for block was to be inserted.
Randomization was achieved by computer generated random number table. Random group were assigned by sealed opaque envelope which was opened by anaesthesiologist not involved in the study after patient was shifted inside operation theatre. The doctor who collected the peri-operative data was blinded to the drug solution administered.
Preoperatively, technique, advantages and risk of anaesthesia was explained to the patient, they were educated about the Visual Analogue Scale (VAS) and then informed consent was taken. Patients were kept nil orally for atleast eight hours prior to surgery and no premedication was given. In the operation theatre intravenous (i.v.) line was secured with 20-gauge cannula in the nonoperated arm and 5 ml/kg/h infusion of 0.9% sodium chloride solution was started. After standard anaesthesia monitoring, baseline measurements of Heart Rate (HR), Noninvasive Arterial Blood Pressure (NIBP), Peripheral Oxygen Saturation (SpO2), and respiratory rate were recorded before the block was performed.
After proper positioning of patient i.e., head is turned to face the opposite direction and the arm abducted to 900, the axillary artery was palpated after preparation of the area and a skin wheal was raised using 2 ml of lidocaine 2%. A short beveled needle inserted over the pulse toward the axilla resulted in a characteristic ‘click’ as it penetrated the sheath. The needle was then allowed to oscillate with the pulse beat. One half of the solution was injected anterior and one half posterior to the artery.
Patients were randomly allocated using a sealed envelope technique and Group L (n=35) received 23 ml of 2% lignocaine with adrenaline (xylocaine 2% with adrenaline 1 in 200000, AstraZeneca, UK Ltd.) + 7 ml of saline, Group LD0.5 (n=34) received 23 ml of 2% lignocaine with adrenaline + 0.5 μg/kg of dexmedetomidine (Dextomid 100μg/1 ml by Neon Laboratories) diluted in saline to make a volume of 7 ml, Group LD1 (n=35) was given 23 ml of 2% lignocaine with adrenaline +1 μg/kg of dexmedetomidine (Dextomid 100 μg/1 ml by Neon Laboratories) diluted in saline to make total volume of 7 ml, the total volume of drug being 30 ml in each group and concentration of lignocaine 1.5%.
Onset, quality and duration of sensory and motor block was assessed. For sensory loss assessment, we used pin prick test with a three-point scale [9]. 0- no effect, 1-analgesia (loss of pinprick sensation), 2-loss of touch in the distribution of median, ulnar and radial nerve. Motor block was assessed by modified Bromage scale [12] for upper extremities using a 3 point scale. 0- complete movement of fingers and wrist, 1- ability to move the fingers only, 2-inability to move fingers.
Evaluation of block was done every three minutes up to 30 minutes after the injection of local anaesthetic. Further block assessment was done by blinded anaesthesiologist at hourly intervals up to 24 hours.
Time interval between the end of injection and complete sensory blockade/complete motor paralysis of wrist and hand was taken as onset of sensory and motor blockade respectively.
Time interval between sensory blockade and reappearance of pinprick response was taken as duration of sensory blockade. Time interval between maximum motor blockade and complete movement of wrist and finger was taken as duration of motor blockade.
Pain assessment was done by using Visual Analogue Scale (VAS).
0 – No pain (one extreme), 10 – Worst pain possible (other extreme)
Patients with VAS > 4 were given injection diclofenac 1.5 mg/kg intramuscularly. If there was no improvement following first rescue analgesic within 30 minutes second rescue analgesic was given in the form of injection tramadol (2 mg/kg).
Two criteria were taken to assess the quality of block - number of partial/failed blocks (inadequate sensory and motor blockade beyond 30 minutes following the infiltration) and surgeon’s satisfaction score based upon ease of performing the surgery and amount of muscle relaxation expressed as VRS (Verbal Response Score) ranging between 0-10. Score 0 for full satisfaction and score 10 for complete dissatisfaction.
Patients were monitored perioperatively for haemodynamic stability and for any side effects. Assessment of sedation was done by Ramsay sedation score [13]. HR and Mean Arterial Blood Pressure (MAP) were recorded at 0 minutes, 5 minutes, 10 minutes, 15 minutes, 30 minutes, 45 minutes, 60 minutes, 90 minutes, and 120 minutes. Adverse events comprised bradycardia was defined as a decrease in HR by 20% from the baseline value or an absolute HR <50 beats per min; which was managed by 0.6 mg IV bolus of atropine, hypotension was defined as fall in blood pressure by 20% from the baseline or an absolute MAP<60 mmHg; which was managed by a bolus of i.v. crystalloids or increments of mephentermine 3 mg i.v. and hypoxemia (SpO2<90%) and managed by supplemental oxygen@ 4 I/min by oxygen mask .
Sample size calculation was based on a pilot study of 15 patients done with similar drug formulation and observed that for projected difference of 20% in duration of postoperative analgesia between the groups, with a type I error of 0.05 and a power of 0.8, 28 patients were required per group. Therefore, to account for the probable drop outs and block failure, we planned to recruit 35 patients in each group, but only 34 patients could be taken in Group LD0.5
Statistical Analysis
Statistical analysis was performed using SPSS software 15.0 (SPSS,Inc.,Chicago, IL) and data was collected and entered in MS Excel 2007. The One-Sample Kolmogorov-Smirnov test was used to determine whether data sets differed from a normal distribution. Normally distributed data was analyzed using a repeat-measures general linear model analysis of variance (ANOVA), whereas non-normally distributed data were interpreted using the Mann–Whitney U-test and categorical data was studied using the Chi-square test. The Bonferroni correction was implied to correct multiple testing at different time points. The p<0.05 was considered significant.
Results
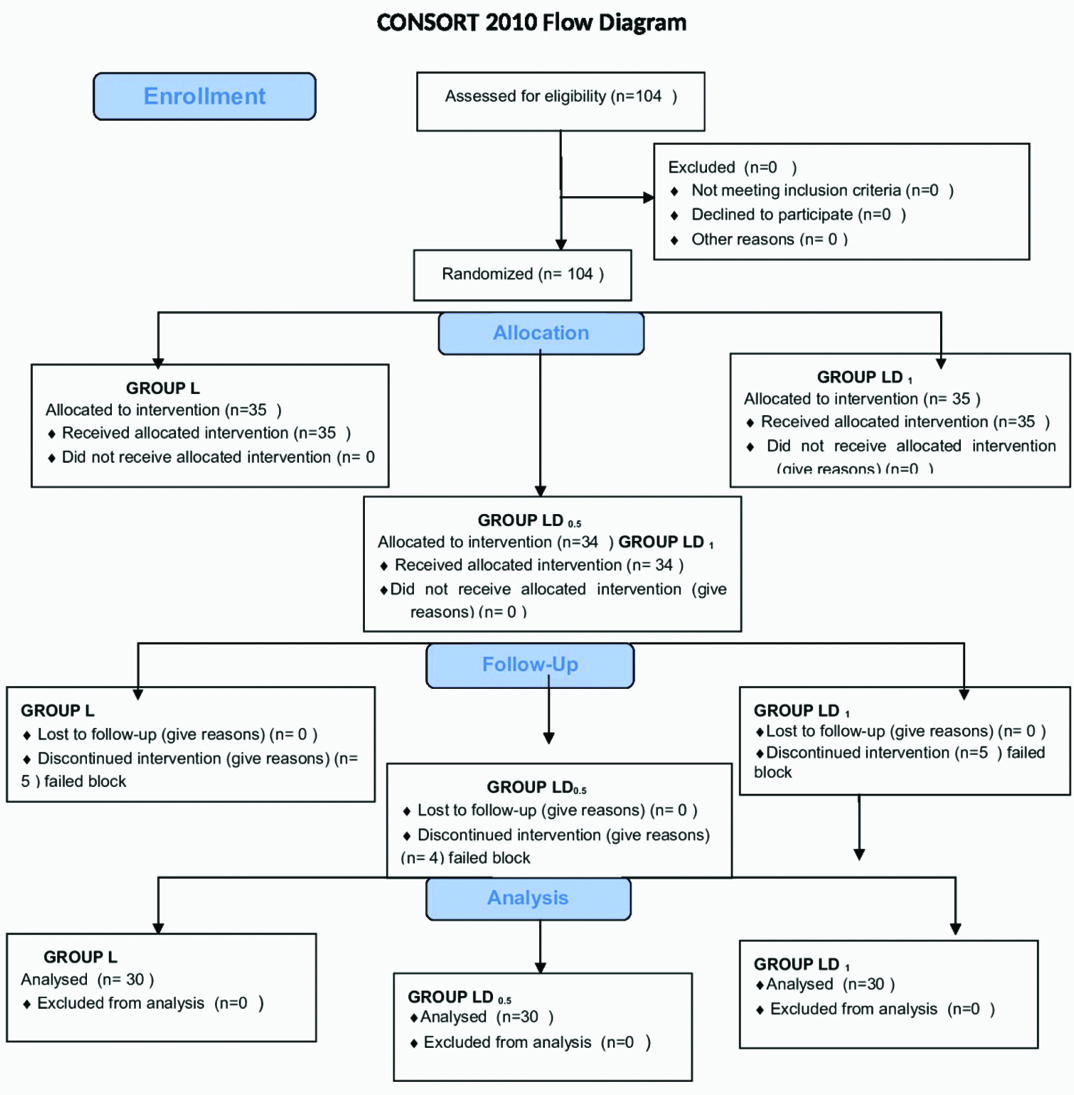
Total number of 104 patients was enrolled during study period. The number of patients who had partial or failed blocks were five in Group L, four in Group LD0.5 and five in Group LD1 [Table/Fig-1]. After excluding these patients the total numbers of patients taken for study were 30 in each group, being comparable to each other with respect to age, gender, weight and duration of surgery [Table/Fig-2].
Demographic data of patients.
| Parameters | L | LD0.5 | LD1 |
|---|
| Age (yrs)* | 29.27(11.6) | 34.27(15.37) | 32.56(13.26) |
| Weight (kgs)* | 51.33(8.98) | 48.37(10.48) | 50.77(10.64) |
| Gender (M/F) | 18/12 | 19/11 | 21/9 |
| ASA I/II† | 20/5 | 20/5 | 18/7 |
| Duration of surgery (mins)* | 69.50(15.1) | 76.67(29.37) | 80.67(19.45) |
| Mean HR (beats/min.)* | 82.1(9.6) | 82.8(9.2) | 80.6(8.9) |
| Mean MBP (mmHg)* | 91.5(9.9) | 92.3(8.9) | 90.6(9.7) |
| Mean SpO2* | 99.6(0.89) | 99.2(1.1) | 99.3(1.0) |
Values expressed as mean ±SD and number as appropriate, No statistical significant difference among the three groups (p>0.05) *ANOVA (analysis of variance), †Chi-square, M = male, F = female, ASA = American Society of Anesthesiologists physical status, HR = Heart Rate, MBP = Mean blood pressure, SpO2 = Percentile oxygen saturation.
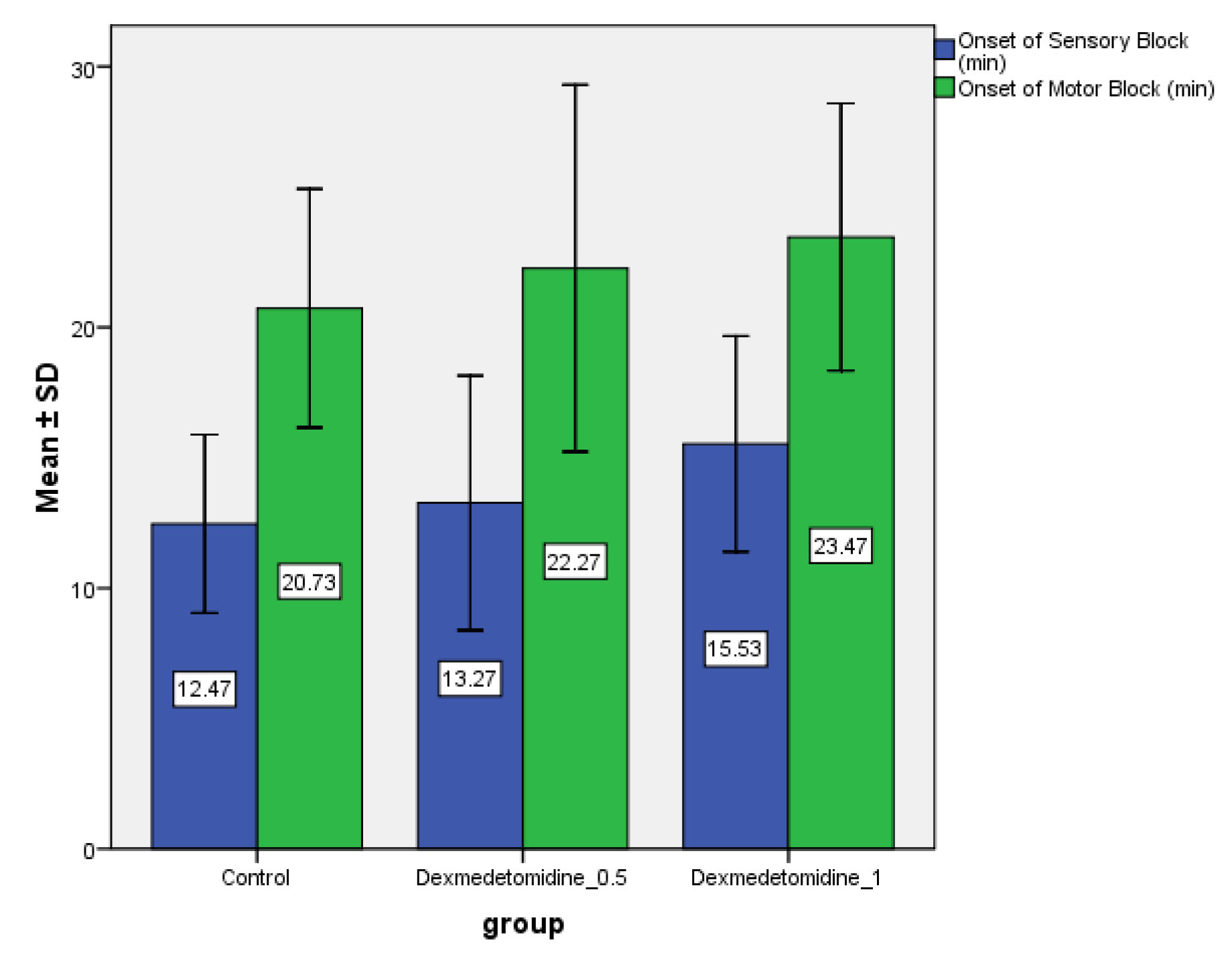
It was observed that onset of sensory block was faster in Group L than Group LD0.5 (p>0.05) and Group LD1 (p<0.01). There was no significant difference in the onset of sensory block in Group LD0.5 as compared to LD1 (p>0.05) [Table/Fig-3]. The onset time for motor block being earlier in Group L (20.73±4.58) minutes as compared to group LD0.5(22.27±7.04) minutes (p>.05) and Group LD1(23.47±5.12) minutes (p<0.05), there was no significant difference in the onset of motor block in Group LD0.5 as compared to LD1 (p>0.05) [Table/Fig-3]. The mean duration of motor block was maximum in Group LD1 (275.43±40.10) minutes followed by Group LD0.5 (201.57±46.40) and minimum in Group L (82.50±21.04) minutes (p< 0.05), whereas the mean duration of sensory block was maximum in Group LD1 (336.03 ± 38.66) followed by Group LD0.5 (252.40±57.13) and minimum in Group L (113.90±19.16) minutes, (p<0.05) [Table/Fig-4].
Comparison of onset of sensory and motor block (minutes) in three groups.
Values expressed as mean±SD.
Comparison of duration of sensory, motor block along with duration of analgesia in three groups. Values expressed as mean±SD. The mean duration of sensory, motor block and analgesic duration was significantly increased in Group LD1 as compared to other groups.
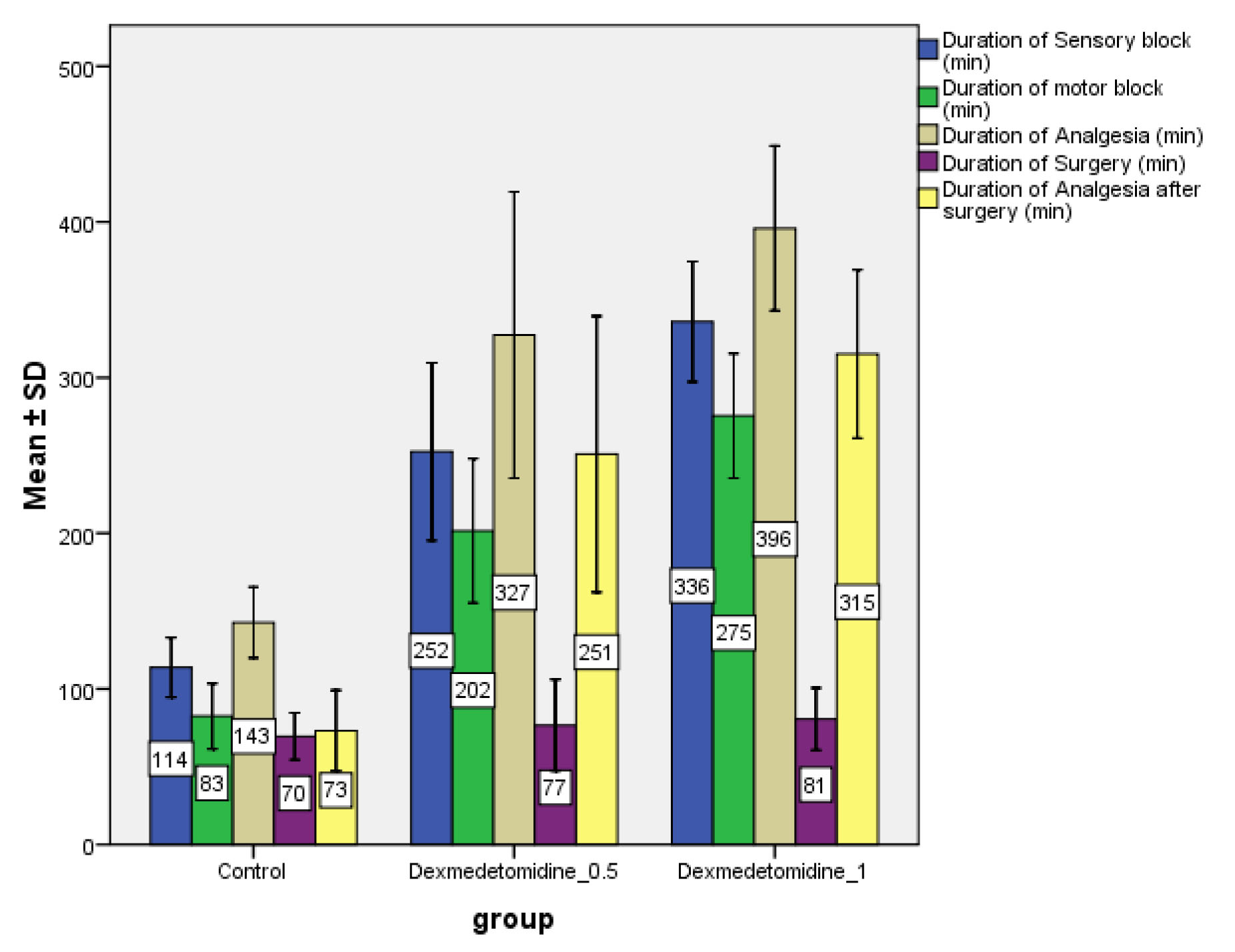
The mean duration of analgesia was maximum in Group LD1 (395.90±52.89) followed by Group LD0.5 (327.30±92.01) and minimum in Group L (142.67±22.78). These durations were significant statistically (p<0.05) [Table/Fig-4].
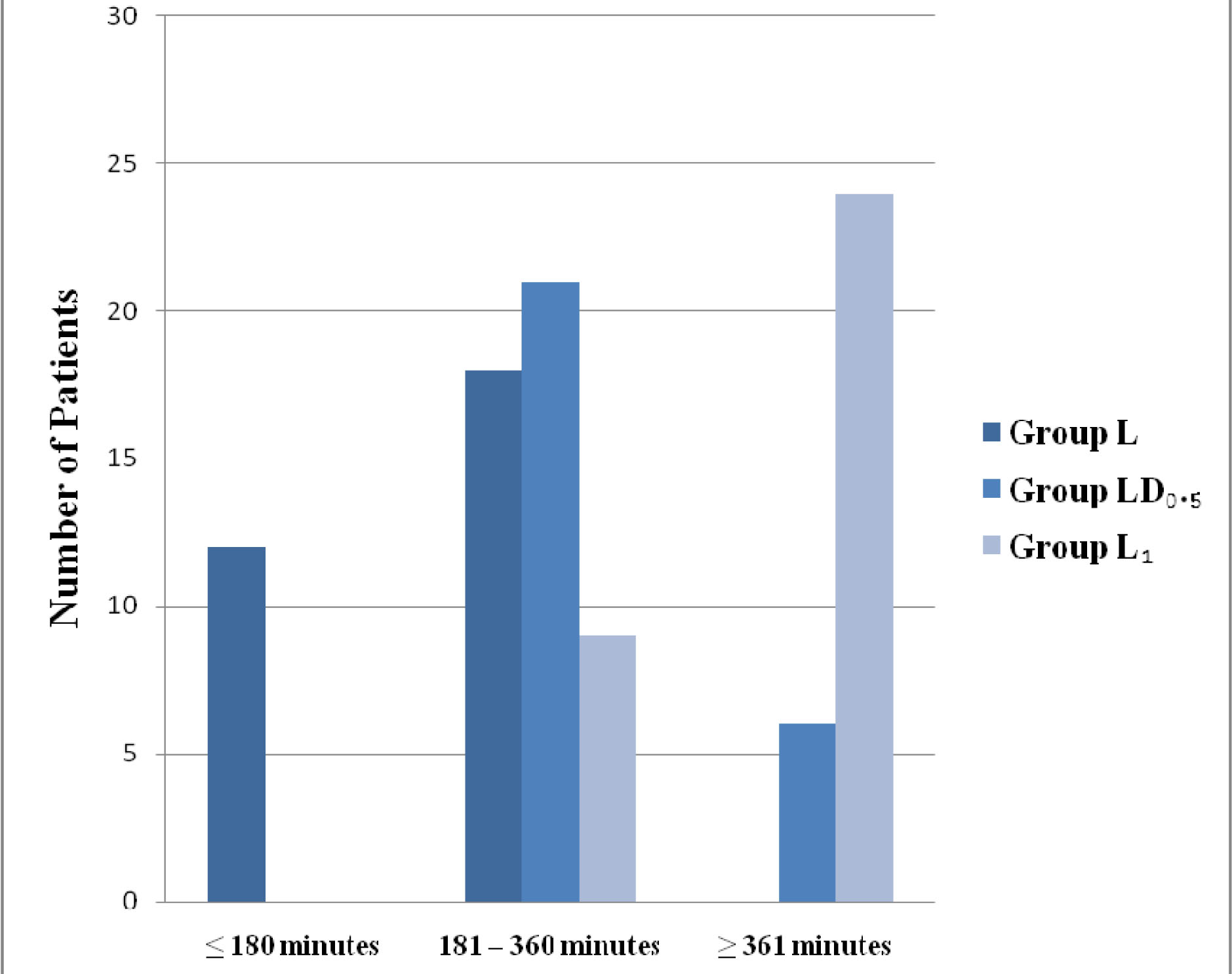
Twelve patients required rescue analgesia in the form of injection diclofenac in Group L in first three hours after completion of surgery; however none of it required it in other two Groups. In the next three hours (181-360) minutes, the number of patients requiring analgesia were 18, 21 and 6 (60%, 70%, 20%) in the Group L, LD 0.5 and LD1respectively. The demand for rescue analgesia was 30% in Group LD0.5 and 80% in Group LD1 beyond six hours of surgery, whereas in Group L all of the patients have required analgesia prior to six hours. Therefore the requirement for first analgesic was considerably delayed in Group LD 1 as compared to other two Groups (p<0.05), [Table/Fig-5]. Twenty four patients in Group L, 25 and 26 patients in Group LD0.5 and Group LD1 respectively had surgeon satisfaction score of 0 (VRS=0) (p>0.05.)
Time for rescue analgesic requirement. Values are expressed as numbers (chi-square test).
Twelve patients in Group LD1 had mild sedation however, they were comfortable throughout the surgery with Ramsay sedation score 3 (score 3 = patient responds to command only) [Table/Fig-6]. Patients remained haemodynamically stable throughout perioperative period with no episode of desaturation.
Comparison of intraoperative sedation scores amongst the three groups.
| Group | SS-B | SS-15 | SS-30 | SS-45 | SS-60 | SS-75 | SS-90 | SS-105 | SS-120 |
|---|
| L | 2.0(0.0) | 2.0(0.0) | 2.05(0.2) | 2.0(0.0) | 2.0(0.0) | 2.0(0.0) | 2.1(0.2) | 2.1(0.3) | 2.0(0.0) |
| LD0.5 | 2.0(0.0) | 2.05(0.2) | 2.15(0.3) | 2.3(0.4) | 2.2(0.5) | 2.1(0.3) | 2.1(0.2) | 2.0(0.0) | 2.0(0.0) |
| LD1 | 2.0(0.0) | 2.4(0.5) | 2.5(0.5) | 2.6(0.4) | 2.5(0.5) | 2.4(0.4) | 2.4(0.5) | 2.4(0.4) | 2.3(0.5) |
| p-value | 1.000 | 0.007* | 0.001† | 0.024* | 0.001† | 0.001† | 0.002† | 0.068 | 0.053 |
Values are expressed as mean ±SD. SS (B, 15, 30, 45, 60, 75, 90,105, 120) – sedation score at baseline, 15, 30, 45, 60, 75, 90, 105 120 minutes respectively. Significantly higher sedation score in group LD1 at 15, 30, 45, 60, 75 and 90 minutes as compared to the other two groups. * p value < 0.05 † p value <0.01
Discussion
This prospective, randomized, double blinded study was performed on patients undergoing orthopaedic surgery of the hand/forearm under axillary brachial plexus block via transarterial approach. All the three groups in our study were comparable in respect to mean age, weight and duration of surgery.
We compared two doses of dexmedetomidine 0.5 μg/kg (Group LD0.5) and 1 μg/kg (Group LD1) respectively as an adjuvant to lignocaine with adrenaline (1:200000) with control (Group L) in axillary brachial plexus block. There was statistical difference in the onset of sensory and motor blockade in the control and LD1 groups, being earlier in control group. Our study demonstrates that the patients who received dexmedetomidine had significantly increased duration of sensory blockade, motor blockade and postoperative analgesia as compared to the control group. Statistically significant increase in the duration of sensory, motor block was observed in LD1 as compared to LD0.5 and control group. Duration of motor blockade was less than the duration of sensory blockade in all the three groups.
The axillary approach to the brachial plexus is the most popular because of its ease, reliability and safety [4]. There is a high success rate up to 92% in some series [14]. Transarterial approach used in our study seems to be safe despite concerns regarding vascular damage and haematoma formation [15].
Gonzalez AP et al., recommended 23.5 ml of 1.5% lignocaine for perivascular injection in ultrasound guided axillary brachial plexus block as minimum effective dose of lignocaine to produce adequate anaesthesia [16]. A 1.5% lignocaine with adrenaline have been successfully used in axillary brachial plexus block in various studies [17] with adequate anaesthesia during intraoperative period.
We chose lignocaine with adrenaline (1:200000) 1.5% for our study and total volume of the drug mixture being 30 ml, so as not to exceed the maximum permissible dose of lignocaine with adrenaline (7 mg/kg) and to quantify the block better with the addition of dexmedetomidine as an adjuvant.
Esmaoglu A et al., demonstrated that addition of 100 μg of dexmedetomidine to 0.5% levobupivacaine hastens sensory and motor block as compared to control and also prolongs the duration of postoperative analgesia, however seven patients in dexmedetomidine group had bradycardia [18]. We, therefore with the intention to recommend a safe optimal dose of dexmedetomidine, chose two doses i.e., 0.5 μg/kg and 1 μg/kg in a controlled trial. The maximum dose which we gave was 75 μg in Group LD1. None of our patient had any side effect like bradycardia, hypotension, nausea, vomiting etc. Time to rescue analgesia was significantly increased in dexmedetomidine group, being longest in LD1 Group. These results were consistent with the findings of Esmaoglu A, however onset of sensory and motor blockade was neither clinically nor statistically significant in all the three groups. The reason might be choice of local anaesthetic. We have used lignocaine with adrenaline (1:200000) in our study, which itself had early onset of sensory and motor blockade unlike levobupivacaine.
Swami SS et al., compared dexmedetomidine with clonidine as an adjunct to 0.25% bupivacaine (35 cc) in supraclavicular brachial plexus block [19]. They used 1 μg/kg of dexmedetomidine and found that dexmedetomidine significantly enhanced the duration of sensory and motor block and also the duration of analgesia. The duration of motor block 472.24±90.06 minutes was more than sensory block 413.97±87.13 minutes in their study, being inconsistent with our study where duration of motor block 275.43±40.10 minutes was less than sensory block 336.03±38.66 minutes with a same dose of dexmedetomidine i.e.,1 μg/kg. Differential blockade is more profound with bupivacaine as compared to lignocaine so this parity in the duration of sensory and motor blockade can be explained on the basis of local anaesthetic used.
Ammar AS and Mahmoud KM compared 0.75 μg/kg of dexmedeto-midine added to 0.33% bupivacaine (30 ml) with control in ultrasound guided single injection infraclavicular brachial block [20]. There was a statistically significant shorter time to onset of sensory blockade (13.2 vs 19.4 minutes), longer duration of sensory block (179.4 vs 122.7 minutes), shorter onset time to achieve motor block (15.3 vs 22.2 minutes), longer duration of motor block (155.5 vs 105.7 minutes) and prolonged analgesia (403 vs 233 min). Shorter duration of motor block (155 minutes) as compared to sensory block (179 minutes) and prolonged analgesia with dexmedetomidine correlates well with our study.
In another study, the addition of dexmedetomidine (100 μg) to 0.375% bupivacaine in brachial block resulted in shorter onset times for sensory and motor blocks (p<0.001) [10], while the duration of blocks was significantly longer (p<0.001) in SD group. The mean duration of analgesia (DOA) for Group SD was 776.4 ± 130.8 min, it was 241.4 ± 51.2 minutes for Group S (p<0.001) with one episode significant bradycardia in SD group . The reason might be choice of local anaesthetic. We have used lignocaine with adrenaline (1:200000) in our study, which itself had early onset of sensory and motor blockade unlike 0.375% bupivacaine.
Till date only single study has evaluated the dose response of dexmedetomidine as an adjuvant to 0.5% lignocaine in IVRA [11] and concluded that the addition of 1 μg/kg dexmedetomidine to lignocaine for IVRA showed significantly better improvement in the quality of anaesthesia and postoperative analgesia in comparison to 5 μg/kg dexmedetomidine, without causing any significant side-effects, as the VAS scores were less in the former group. So, the authors advocated to use dexmedetomidine at a dose of 1 μg/kg as an adjunct to lignocaine in IVRA for upper limb surgeries. However, the mean onset of sensory and motor block was significantly earlier in 1 μg/kg group (p<0.001), whereas in our study the mean onset of sensory (p<0.05) and motor block (p>0.05) was inversely related to the dose i.e., with increase in dose there was increase in onset time. The reason might be use of 1.5% lignocaine in our study in comparison to 0.5% lignocaine in the above said study. We didn’t encounter any side effect i.e., hypotension and bradycardia in any of three groups. The patients in LD1 Group were sedated and responded to verbal commands (RSS=3) without any episode of desaturation or bradycardia.
However, in our study, there was significant increase in duration of postoperative analgesia with 1 μg/kg dexmedetomidine as compared to 0.5 μg/kg dose and control group, which led to less consumption of nonsteroidal anti-inflammatory drugs in the postoperative period.
Limitation
The major limitation of our study was the non availability of ultrasound. Use of ultrasound would have further decreased the volume and dose of anaesthetic used. Neither we had systemic control which could have made us to comment on the peripheral action of dexmedetomidine. But there are studies which have already proved this and our main aim was to comment on safe optimal dose of dexmedetomidine which could be used as an adjuvant to lignocaine.
Conclusion
The use of dexmedetomidine 0.5 μg/kg and 1 μg/kg as an adjuvant to lignocaine with adrenaline (1:200000) in transarterial axillary brachial plexus block increases the duration of sensory, motor blockade and decreases as well as delays the requirement of rescue analgesia postoperatively without causing any side effects as compared to control group, maximum benefit being observed with addition of 1 μg/kg dexmedetomidine.
We therefore recommend that 1 μg/kg dexmedetomidine has better therapeutic profile as compared to 0.5 μg/kg and control group, without any significant side effects, as an adjuvant to lignocaine with adrenaline in axillary brachial plexus block.
Values expressed as mean ±SD and number as appropriate, No statistical significant difference among the three groups (p>0.05) *ANOVA (analysis of variance), †Chi-square, M = male, F = female, ASA = American Society of Anesthesiologists physical status, HR = Heart Rate, MBP = Mean blood pressure, SpO2 = Percentile oxygen saturation.
Values are expressed as mean ±SD. SS (B, 15, 30, 45, 60, 75, 90,105, 120) – sedation score at baseline, 15, 30, 45, 60, 75, 90, 105 120 minutes respectively. Significantly higher sedation score in group LD1 at 15, 30, 45, 60, 75 and 90 minutes as compared to the other two groups. * p value < 0.05 † p value <0.01