Myofibroma is a benign uncommon fibroblastic tumour originating from the soft tissue, bone and may affect both children and adults. Radiographically myofibroma presents as unilocular radiolucency. Histologically, typical biphasic cellular arrangement is noted. Immunohistochemical markers are useful for definitive diagnosis of this uncommon neoplasm. Here, a case of six-year-old male child with a localized swelling in the left body of mandible is presented. The clinical, radiological, histological and immunohistochemical findings were corroborative of Infantile Myofibroma.
Case Report
A six-year-old male child patient reported in the Department of Oral and Maxillofacial Pathology of Guru Nanak Institute of Dental Sciences and Research with the chief complaint of swelling involving left side of the lower jaw since two months. There was a history of trauma two and half months ago. No history of fever, weight loss or other constitutional symptoms was noted.
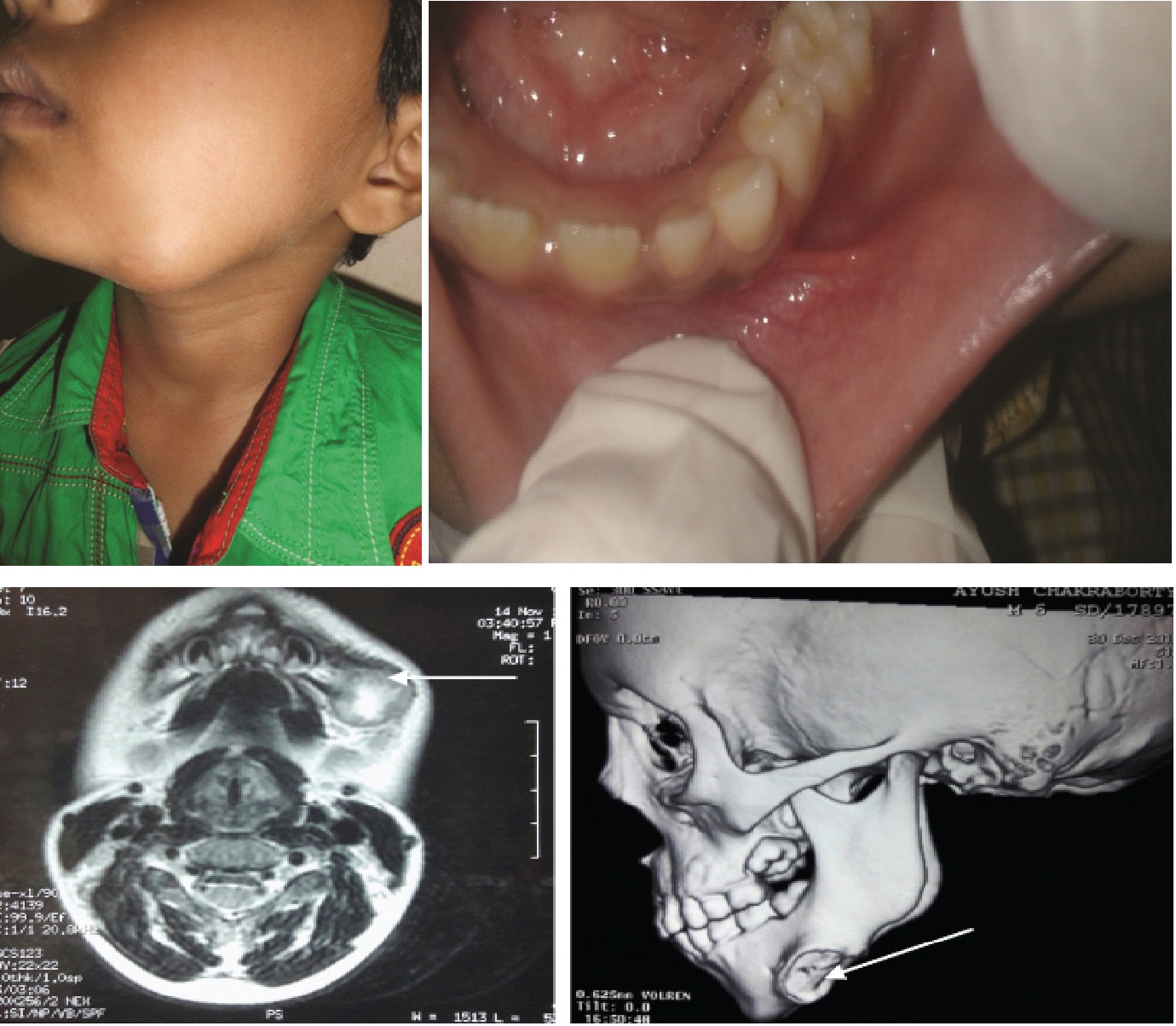
The swelling was initially noticed as a small nodule which increased gradually to attain the present dimension. Extraorally there was presence of a well demarcated swelling involving the left side of the body of mandible [Table/Fig-1a]. On palpation the swelling was well localized, firm, non mobile, 2x2 cm in diameter, non tender, fixed to the underlying bone and non adherent to the superficial skin. No submandibular lymphadenopathy was noted. Intraorally no abnormality was detected [Table/Fig-1b].
Orthopantomogram revealed no abnormal features within the tooth bearing areas and jaw bones except for a soft tissue shadow visible in relation to permanent tooth bud of left mandibular canine to second molar region. PA view of chest revealed no abnormal features.
Magnetic Resonance Imaging (MRI) evaluation of the maxillofacial region, comprising axial, sagittal and coronal T2- weighted imaging showed signal changes involving left submandibular region with areas of necrosis and soft tissue swelling with oedema. Mild signal change was also seen from adjacent bony portion. Plain Computed Tomography (CT) scan of the jaws with 3D reconstruction image showed the presence of a soft tissue Space Occupying Lesion (SOL) with erosion of bone in relation to the inferior aspect of left side of the body of the mandible [Table/Fig-1c,d].
Based on the above clinical and radiological features, a provisional diagnosis of benign neoplasm of fibrous, neural or muscular tissue origin was made and the patient was referred to the Department of Oral Surgery for surgical maneuver.
An incisional biopsy was planned and routine blood test was found to be within normal limits. After written informed consent from the patient and ethical clearance, an incisional biopsy was performed under general anesthesia and the specimen was sent for histopathological evaluation.
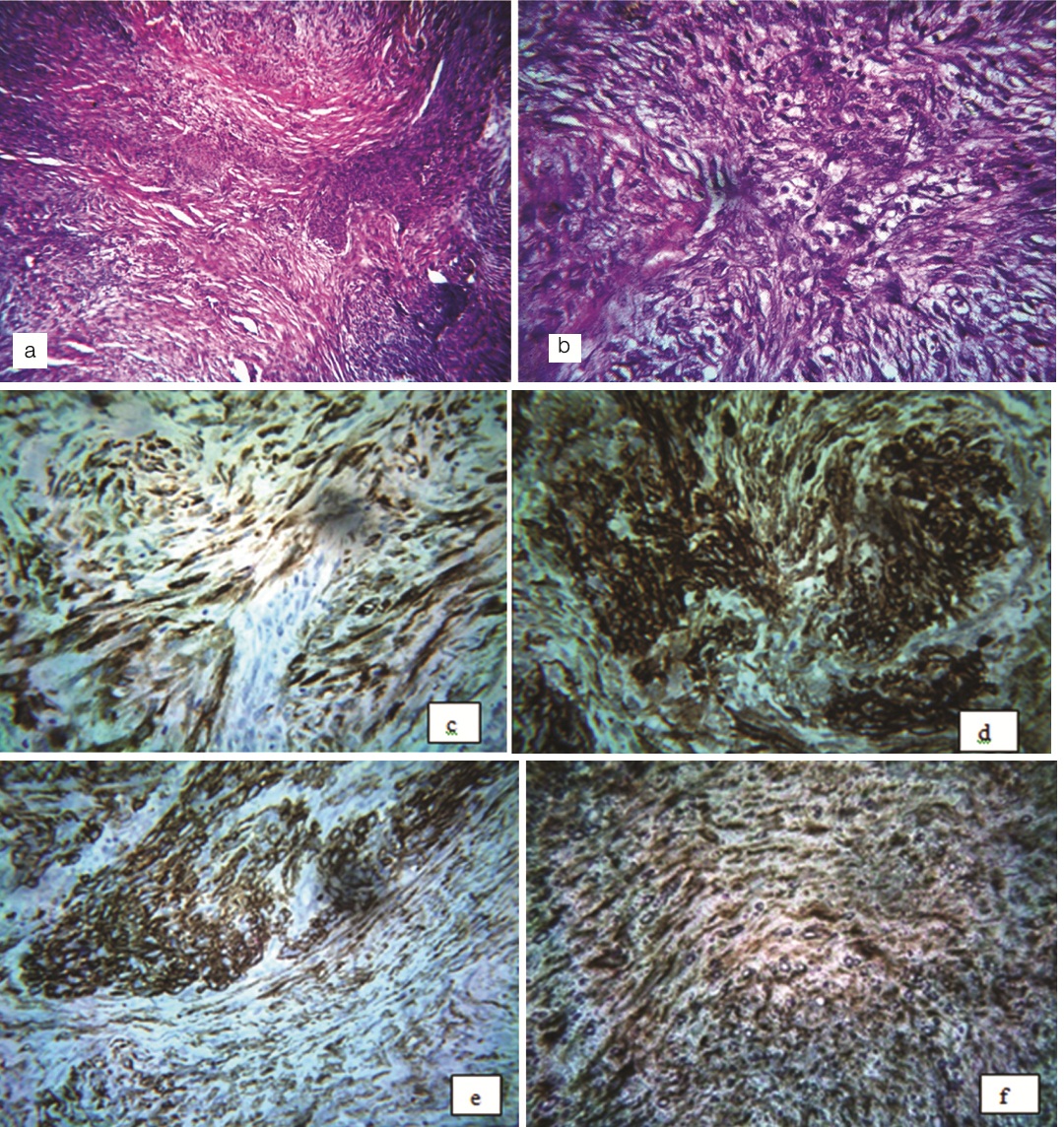
Histopathological examination revealed the presence of tumour tissue composed of stromal cells exhibiting biphasic pattern with light and dark staining areas. The light staining areas mainly consisted of spindle cells with eosinophilic cytoplasm arranged in fascicular or storiform pattern with elongated nuclei. These areas often alternate with closely packed round cells with slightly pleomorphic hyperchromatic nuclei, around distinct haemangiopericytoma like vascular spaces. Cellular atypia was not noted. The histopathological diagnosis of ‘Myofibroma’ was made and to confirm this immunohistochemical analysis of a panel of antibodies was done. Strong immunopositivity was noted for Smooth Muscle Actin (SMA), Muscle Specific Actin (MSA), vimentin and calponin, whereas negative immunoreactivity was noted for p-63, desmin, h-Caldesmon. Based on the above clinical, histopathological and immunohistochemical findings, a definitive diagnosis of ‘myofibroma’ was confirmed [Table/Fig-2].
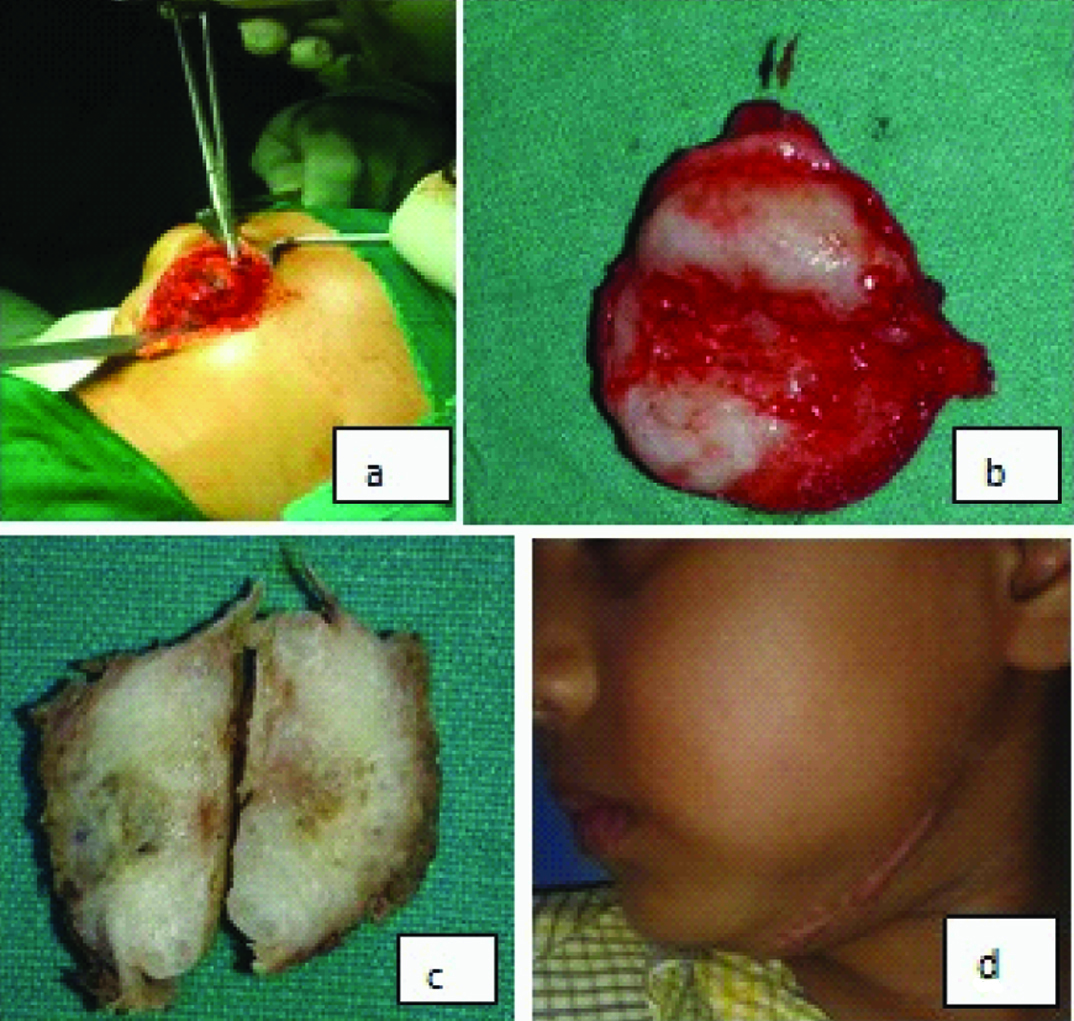
Then the patient was again sent to the Plastic Surgery Department of RG Kar Medical College Hospital, Kolkata for further surgical treatment. In Oral Surgery Department the lesion was surgically explored under general anaesthesia. A 1.5 inch horizontal incision was given near the left submandibular region. After blunt dissection the tumour mass was exposed, this was attached to the left body of the mandible [Table/Fig-3a]. Excision of the tumour mass was done along with removal of a portion of regional bony margin.
The excised specimen was greyish white, multilobulated, having gelatinous surface which was further sent to our department for histopathological evaluation [Table/Fig-3b,c]. Stained section from the excised specimen also revealed the characteristic confirmative features of ‘Myofibroma’. The patient was followed up at regular interval and no signs of recurrence were noted over the period of 12 months [Table/Fig-3d].
a) Extraoral; and b) Intraoral photographs of the patient; c) Magnetic Resonance Imaging (MRI) revealed the presence of signal changes in left submandibular region (arrow); d) Plain Computed Tomography (CT) scan of the jaws showing erosion of bone in relation to the affected region (arrow).
a) – Low power photomicrograph showing biphasic appearance of the tumour cells (H&E 10X); b) High power photomicrograph showing spindle shaped cells arranged in storiform pattern with elongated nuclei along with presence of round cells (H&E 40X). Immunohistochemistry (40X) showing the positivity to c) Calponin; d) MSA; e) SMA; and f) Vimentin.
a) Photograph showing surgical exposure of the tumour mass; b) The excised tumour mass; c) The cut surface of the tumour; d) Extraoral photograph showing the 12 months postoperative view of the patient.
Discussion
Myofibroma and myofibromatosis are rare benign mesenchymal neoplasms originating from myofibroblast which may occur in the soft tissue, bone or internal organs [1,2]. They were first described by Stout AP under the term “Congenital generalized fibromatosis” [3]. Smith KJ et al., in 1989 reported a solitary case of this tumour under the term ‘myofibroma’ following which World Health Organisation adopted the term myofibroma (solitary) and myofibromatosis (multicentric) to describe benign neoplasm of contractile myoid cells [4], though myofibroma commonly occur in the head neck region, however intraosseous lesions are rare. It is most commonly seen in children between birth upto 12 years of age [5].
The cause of myofibroma is currently unknown, though trauma has been implicated in the pathogenesis of this neoplasm. In response to trauma or any mechanical tension the fibroblast cells get stimulated and under the influence of platelet derived growth factor they are differentiated into protomyofibroblast. In the cytoplasm of this cell, accumulation of more amounts of actin microfilaments (stress fibres) occurs. Then under the influence of Transforming Growth Factor- beta (TGF-β) and EDA-Fibronectin these protomyofibroblast cells are differentiated into myofibroblast cells. The proliferation of these myofibroblast is responsible for occurrence of myofibroma [5]. A history of trauma was noted in the present case which could have caused the proliferation of myofibroblast.
Clinically the diagnosis of myofibroma is challenging, as the lesion presents as an asymptomatic, firm, nodular swelling which may cause expansion of jaw bones leading to facial asymmetry [2,5,6]. In the present case a firm, non mobile mass was noted over the left side of the body of the mandible in a six-year-old child which is in accordance with the clinical findings reported in various studies [5-7].
Radiographically, myofibromas usually appear as unilocular or multilocular radiolucency surrounded by a thick sclerotic border. Some cases show thining of cortical plate along with displacement of regional teeth [5]. MRI is useful specially when the soft tissue tumours are present. CT scan is helpful to diagnose any bony erosion/involvement [8]. Our case did not show any characteristic radiological feature on OPG but significant bony erosion was noted on CT scan and MRI imaging technique.
Histopathology reveals the presence of spindle shaped cells arranged in intersecting bundles, which are uniform, having vesicular nuclei, eosinophilic cytoplasm and indistinct cell borders. There is also presence of numerous blood vessels along with the proliferation of round cells giving haemangiopericytoma like appearance. The dark staining area with proliferation of round cells and light staining areas consisting of spindle cells gives the tumour a biphasic appearance [1,5,6,8-11]. This alternating light and dark stained area creates a micronodular ‘Zoning’ phenomenon, which is characteristic for myofibroma. These features were also evident in the present case [6,7,12]. Myofibroma shows positive immunoreactivity to vimentin, α-smooth muscle actin and muscle specific actin and immunonegativity to desmin, S-100 and CD-34 [1,2,6-9,12]. In our case immunopositivity was found in SMA, MSA, calponin and vimentin whereas desmin, h-Caldesmon and p63 showed immunonegativity. The histopathological differential diagnosis of myofibroma includes the tumours of muscle origin, neural origin and other tumours like desmoplastic fibroma, fibromatosis and low grade fibrosarcoma. The neoplastic cells of leiomyoma are positive for desmin in contrast to myofibroma. Schwannoma and neurofibroma do not contain hemangiopericytoma like blood vessels and shows immunopositivity for S-100 protein whereas myofibroma shows negativity for S-100. The absence of haemangiopericytoma like vascular pattern can help to differentiate desmoplastic fibroma from myofibroma. Desmoplastic Fibroma (DF) also shows less constant positivity to α-SMA whereas myofibroma shows strong positivity to it. Fibromatosis has a more monomorphic growth pattern in comparision to characteristic biphasic pattern of myofibroma [2]. Evaluation of desmin expression helps to distinguish myofibroma from myofibrosarcoma. Uniformly negative espression is seen in myofibroma, whereas strong immunopositivity to desmin is seen in myofibrosarcoma [1,2,5,7,12].
Treatment for the typical solitary lesion is enucleation to resection, depending on the extent of the lesion [5]. A 7% to 31% of local recurrence has been reported to occur in excised cases of myofibromatosis [12]. The prognosis is good in case of solitary lesion, whereas multiple myofibromatosis have got fatal outcome [1]. Chemotherapy or radiation has been seldom used for myofibromatosis except for a few cases with recurrence or non resectable lesion [1,12,13]. In this case excision of the tumour mass was done along with the removal of a portion of regional bony margin. No signs of recurrence have been seen after one year post surgical period.
Conclusion
Myofibroma is a benign, self limiting and localized tumour consisting mainly of myofibroblast. Since the prognosis of this tumour is variable and can range from excellent to sometimes fatal, so early and proper diagnosis is mandatory.
Acknowledgements
The authors gratefully acknowledge the contributions made by Professor (Dr) RR Paul, Deputy Director cum-in-charge, Research and Development, GNIDSR, Kolkata, Professor (Dr) M Pal, HOD, Department of Oral and Maxillofacial Pathology, GNIDSR, Kolkata and Prof. (Dr) Rup Bhattacharya, HOD, Plastic Surgery Department, RG Kar Medical College and Hospital, Kolkata for their valuable guidance and help in every aspect.
[1]. V Narayen, SA Ahmed, C Suri, S Tanveer, Myofibroma of the gingiva: A rare case report and literature reviewCase Reports in Dentistry 2015 2015:243894 [Google Scholar]
[2]. PD Souza, CCS Loureiro, RAG Rejas, SOM Sousa, R Raitz, Intraosseous myofibroma simulating an odontogenic lesionJournal of Oral Science 2009 51(2):307-11. [Google Scholar]
[3]. AP Stout, Juvenile fibromatosesCancer 1954 7:953-78. [Google Scholar]
[4]. KJ Smith, HG Skelton, TL Barrett, GP Lupton, JH Graham, Cutaneous myofibromaMod Pathol 1989 2(6):603-09. [Google Scholar]
[5]. AB Urs, S Mohanty, S Arora, J Augustine, P Kumar, G Malik, Pediatric solitary intraosseous infantile myofibroma of the mandibleJournal of Dentistry for Children 2014 8(1):42-46. [Google Scholar]
[6]. B Rai, E Ludusan, B McGovern, F Sharif, Mandibular swelling in a 5-year-old child- mandibular myofibromaBMJ Case Rep 2014 2014.pii: bcr2014203977 [Google Scholar]
[7]. S Sundaravel, K Anuthama, H Prasad, HJ Sherlin, Intraosseous myofibroma of mandible: A rarity of jaws: with clinical, radiological, histopathological and immunohistochemical featuresJournal of Oral and Maxillofacial Pathology 2013 17(1):121-25. [Google Scholar]
[8]. A Thennavan, V Narayanaswamy, TM Niazi, L Rao, R Radhakrishnan, Infantile myofibroma eroding into the frontal bone: a case report and review of its histopathologic differential diagnosisCase Reports in Pediatrics 2012 2012:630804 [Google Scholar]
[9]. EB Chung, FM Enzinger, Infantile myofibromatosisCancer 1981 48(8):1807-818. [Google Scholar]
[10]. H Narchi, Four half-siblings with infantile myrofibromatosis: A case for autosomalrecessive inheritanceClinical Genetics 2001 59(2):134-35. [Google Scholar]
[11]. DJ Zand, D Huff, D Everman, F Russell, S Saitta, D McDonald-McGinn, Autosomal dominant inheritance of infantile myofibromatosisAmerican Journal of Medical Genetics 2004 126(3):261-66. [Google Scholar]
[12]. G Guglielmi, L Guida, P Bacchini, LL Muzio, F Bertoni, LL Russo, Imaging study of myofibroma of the jaws: case report and literature reviewOral Radiology 2016 32(3):195-205. [Google Scholar]
[13]. T Sugatani, M Inui, T Tagawa, Y Seki, A Mori, J Yoneda, Myofibroma of the mandible. Clinicopathologic study and review of the literatureOral Surg Oral Med Oral Pathol Oral Radiol Endod 1995 80:303-09. [Google Scholar]