Acute Respiratory Distress Syndrome Complicated by Amiodarone Induced Pulmonary Fibrosis: Don’t Let Your Guard Down
Vipin Kumar Singh1, Vijeta Maheshwari2
1 Assistant Professor, Department of Anaesthesia, King George’s Medical University, Lucknow, Uttar Pradesh, India.
2 Senior Resident, Department of Anaesthesia, King George’s Medical University, Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vipin Kumar Singh, Jewel Apartment A-2-2, Jopling Road, Hazratganj, Lucknow-226003, Uttar Pradesh, India.
E-mail: vipintheazad@gmail.com
Amiodarone is an antiarrhythmic agent which is commonly used to treat both supraventricular and ventricular arrhythmias. This iodine containing compound has been associated with several adverse events like it tends to accumulate in several organs. Among those, the most serious is Amiodarone Pulmonary Toxicity (APT). While the incidence of this complication has decreased with the use of lower doses of amiodarone but it can occur with any dose. Pulmonary complications usually present as an acute or subacute pneumonitis. On chest X-ray and high-resolution Computed Tomography (CT), diffuse infiltrates were found. Here, we present a case in which acute respiratory distress syndrome like features were detected which got subsided after stopping tablet amiodarone. The patient was a known case of atrial fibrillation for which she was taking tablet amiodarone for the last six months.
Arrhythmia, Pulmonary toxicity, Upper respiratory tract infection
Case Report
A 75-year-old female presented with severe respiratory distress which was gradual in onset and progressive in nature. She had complaint of dry cough and running nose for last three days. There was no prior admission for such event and no history of smoking. She was a known case of atrial fibrillation for which she was taking tablet amiodarone 200 mg in the night for last six months. We started I.V. meropenem, teicoplanin and levofloxacin. Due to the deteriorating oxygen saturation and dyspnea, she was put on ventilator with 60 % oxygen with high level of Positive End-Expiratory Pressure (PEEP) in suspicion of Acute Respiratory Distress Syndrome (ARDS) but there was not much improvement in oxygenation.
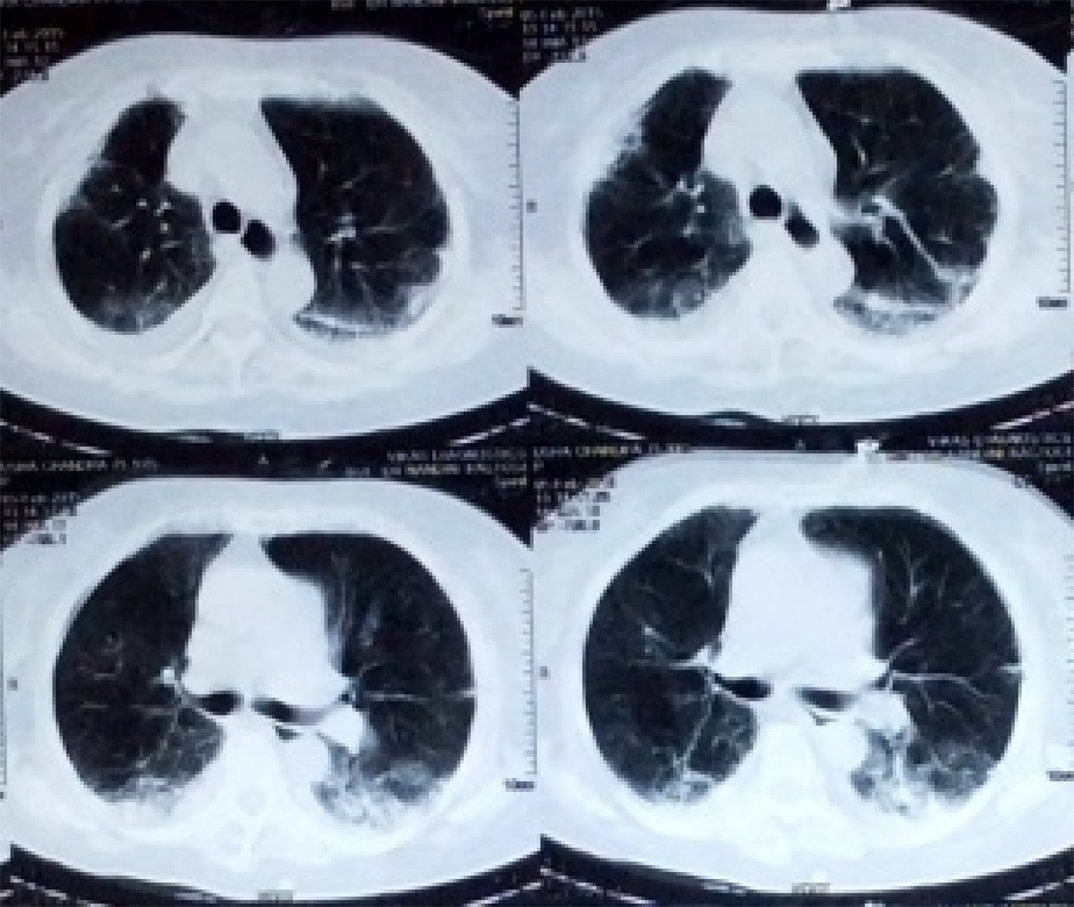
On X-ray chest, there was bilateral diffuse opacity. On third day of ventilatory support, there was slight improvement in oxygenation however, we were unable to decrease ventilator support and thus planned a High Resolution Computed Tomography (HRCT) chest. On HRCT, bilateral lung field showed interstitial fibrosis suspecting interstitial lung disease [Table/Fig-1]. Total leukocyte count and serum procalcitonin were within normal limit. On third day of mechanical support, tablet amiodarone was stopped and changed over to tablet diltiazem to control ventricular rate regarding atrial fibrillation. Injection methyl prednisone was started in suspicion of amiodarone induced pulmonary dysfunction.
CT thorax showing pulmonary fibrosis.
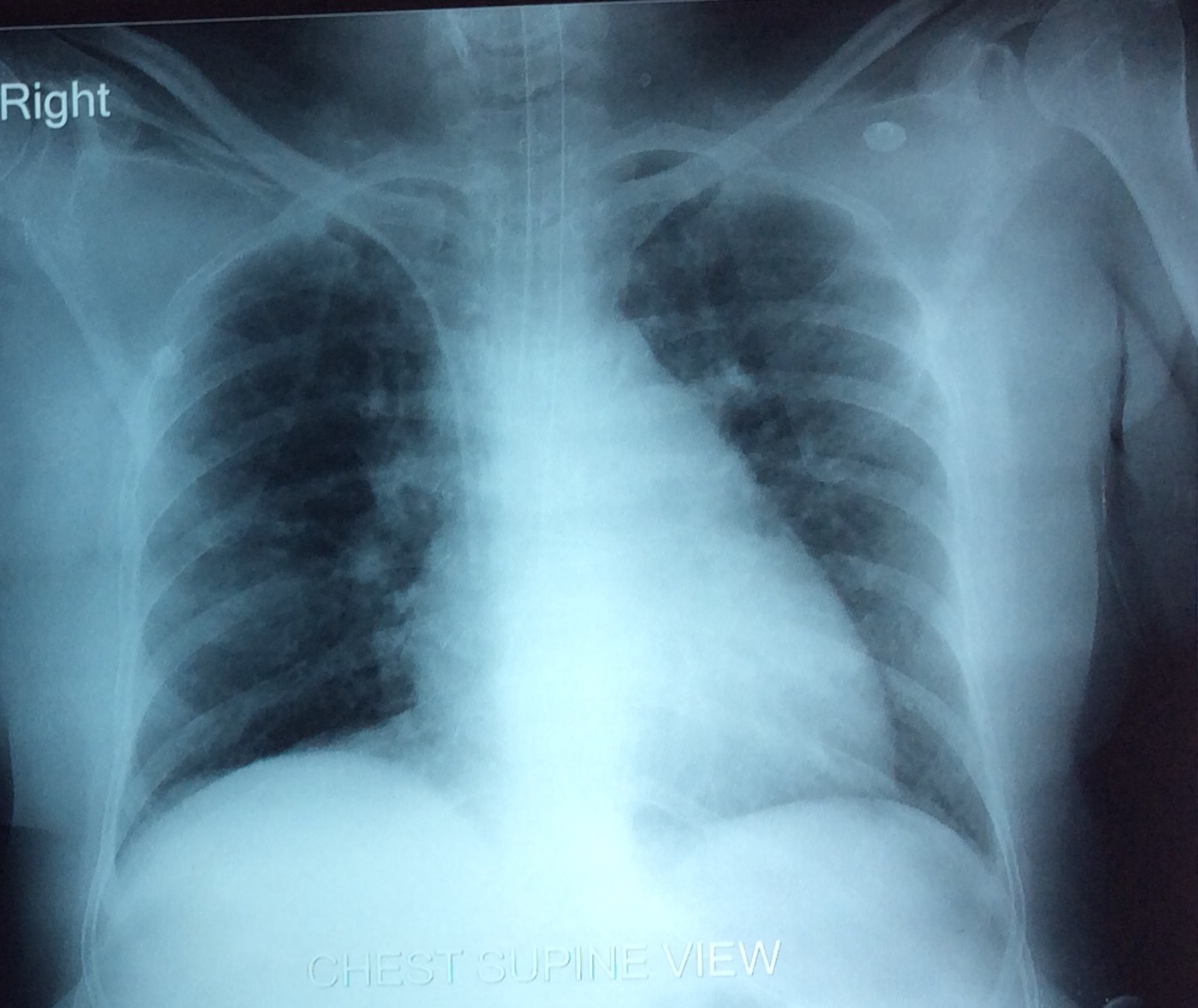
Patient’s condition improved after seven days and extubated on 10th day of ventilator. Chest X-ray showing resolution of opacities [Table/Fig-2]. Oral steroid continued for next one month and then tapered slowly with weekly follow up in OPD. After two month, she was alright except having complaint of dyspnea on exertion.
X-ray chest showing resolution of opacities.
Discussion
Amiodarone is an iodine-containing compound like thyroxine and is an antiarrhythmic agent, commonly used to treat supraventricular and ventricular arrhythmias. It tends to accumulate in several organs, including the lungs. It has got various adverse effects too. Of these events, the most serious is APT.
Bioavailability increases if taken with food. High lipid solubility leads to accumulation in adipose tissue and highly perfused organs such as the liver, lungs and spleen. The large volume of distribution leads to a long elimination half-life (up to six months) [1].
Two major risk factors for APT are age and duration of therapy [2]. Toxicity can occur during or even discontinuation of drug. Patients on highest risk are those taking 400 mg/day cumulatively for greater than two months or 200 mg/day for two years. The risk of toxicity increases with higher plasma concentration. However, APT depends on the total cumulative dose rather than the daily dose or plasma concentration of amiodarone [3]. Amiodarone and its metabolites can produce lung damage directly by a cytotoxic effect and indirectly by an immunological reaction [4].
Diagnosis is often made by exclusion as there are no specific laboratory analyses to confirm APT. It is based on a combination of strong clinical suspicion, history, radiographic and clinical evidence, and the thorough exclusion of alternative aetiologies. Decrease in the diffusion capacity for carbon monoxide is the earliest finding on pulmonary function test. Gallium scanning could be used to differentiate APT from cardiogenic pulmonary oedema, one of the common comorbidities that often confound diagnosis [5]. Bronchoscopy with Bronchoalveolar Lavage (BAL) and transbronchial biopsy are useful to make diagnosis [6].
Typical picture on microscopy in APT shows a diffuse interstitial pneumonitis. This finding is due to hyperplasia of type II pneumocytes and widening of alveolar septae along with inflammatory infiltrates and varying degrees of interstitial fibrosis. Individuals usually present with malaise, fever, progressive shortness of breath, nonproductive cough, and sometimes pleuritic chest pain.
The most remarkable manifestation of APT is that of a rapidly progressive diffuse pneumonitis with acute respiratory failure and a picture simulating ARDS. APT should be suspected in any patient taking amiodarone who have above described respiratory symptoms with new infiltrates on chest X-ray. In vulnerable patients, it may also lead to pulmonary oedema, pneumonia, pulmonary embolism or other acute events, which may make the diagnosis difficult. In the appropriate clinical setting with the findings on chest X-ray and computed tomography scan might be sufficient to support the diagnosis and to initiate treatment [7].
While considering therapeutic options in cases of amiodarone induced toxicity, we must keep in mind that the drug and its metabolite (desethylamiodarone) are not dialyzable [8].
The primary treatment of APT is immediate discontinuation of amiodarone but even after discontinuation, amiodarone resolution is likely to be slow due to large volume of distribution [9]. Systemic corticosteroids are suggested for the treatment of APT, although controlled trials demonstrating efficacy are lacking. Usually prednisone in doses of 40-60 mg/day orally are used and tapered slowly. Again, the pharmacodynamics of amiodarone dictates that treatment should be continued for 4 to 12 months because cases of relapse on early steroid withdrawal have been reported [7].
Conclusion
Although the incidence of this complication has decreased with the use of lower doses of amiodarone but it remains an important diagnostic consideration due to increased use of this drug. Clinical manifestations of pulmonary toxicity may be subtle but sometimes fatal. With prompt diagnosis, most patients respond favorably to the withdrawal of amiodarone and the administration of corticosteroids. A baseline chest X-ray and pulmonary function study should be performed before starting amiodarone, and all patients should be followed carefully and monitored appropriately to ensure early diagnosis of pulmonary toxicity.
[1]. Vassallo P, Trohman RG, Prescribing amiodaroneJAMA 2007 298:1312-22. [Google Scholar]
[2]. Ernawati DK, Stafford L, Hughes JD, Amiodarone-induced pulmonary toxicityBr J Clin Pharmacol 2008 66:82-87. [Google Scholar]
[3]. Jessurum GA, Crijns HJG, Amiodarone pulmonary toxicityBMJ 1997 314:619-20. [Google Scholar]
[4]. Okayasu K, Takeda Y, Kojima J, Amiodarone pulmonary toxicity: A patient with three recurrences of pulmonary toxicity and consideration of the probable risk of relapseIntern Med 2006 45:1303-07. [Google Scholar]
[5]. Fabiani I, Tacconi D, Grotti S, Amiodarone-induced pulmonary toxicity mimicking acute pulmonary oedemaJ Cardiovasc Med (Hagerstown) 2011 12:361-65. [Google Scholar]
[6]. Yamada Y, Shiga T, Matsuda N, Incidence and predictors of pulmonary toxicity in japanese patients receiving low-dose amiodaroneCirc J 2007 71:1610-16. [Google Scholar]
[7]. Ott MC, Khoor A, Leventhal JP, Pulmonary toxicity in patients receiving low-dose AmiodaroneChest 2006 123:646-51. [Google Scholar]
[8]. Zimetbaum P, Amiodarone for atrial fibrillationN Engl J Med 2007 356:935-41. [Google Scholar]
[9]. Schwaiblmair M, Berghaus T, Haeckel T, Amiodarone-induced pulmonary toxicity: An under-recognized and severe adverse effect? Clinical Research In Cardiology: Official Journal of the German Cardiac Society 2010 99:693-700. [Google Scholar]