TBM is one of the most severe forms of CNS-TB and is associated with a high frequency of neurological sequelae and mortality if not treated promptly [1]. The estimated mortality due to TBM in India is 1.5 per 100,000 populations [2]. Clinical diagnosis of TBM remains difficult mainly due to unspecific clinical features which varies widely and is often diagnosed when brain damage has already occurred.
Molecular tests such as PCR based upon fragment amplification of genomic sequences of M.tuberculosis (MTB) are currently considered as rapid and a sensitive method for detection of MTB in CSF samples [3,4]. PCR assays based on MTB specific insertion sequences such as IS6110 gene have been investigated in many reports due to its high amplification frequency as these insertion sequences are present in multiple copies in the genome of MTB [3,5,6]. However, both sensitivity and specificity of such PCR assays vary according to different reports and are usually dependent on CSF bacterial load and volume of CSF collected [7,8]. The standard lumbar puncture method commonly known as spinal tap remains the method of choice for CSF collection. The volume of CSF collected by spinal tap may vary according to the patient and the person collecting it [9] and may cause considerable distress and infection in patients if repeatedly collected. Moreover, CSF collection by standard lumber puncture cannot be done in patients having clotting problem or thrombocytopenia, thus creating a major obstacle in final diagnosis and treatment initiation.
To obtain a sensitive detection methodology, in spite of using specimens from the site of infection, some researchers have tried to detect the MTB DNA from a patient’s blood of both PTB and EPTB [10-12], as blood can be easily drawn from the patients. However, there are no studies till dates that determine the presence of MTB in blood in TBM cases.
In the present study, we have compared the diagnostic utility of PCR assay in both peripheral blood and CSF samples. The main objective of our study was to evaluate the application of the peripheral blood PCR assay as an alternate tool for TBM diagnosis compared to conventional CSF-PCR based system.
Materials and Methods
This was a pilot study carried out in the Neurology Department of the CIIMS, Nagpur. A total of 50 cohorts admitted to IPD wards with suspected TBM infection between January 2014 to Feburary 2015 were enrolled in the study. Written consent was obtained from all participants or their kin after oral explanation about the study. All study protocols were approved by the Institutional Ethics Committee of CIIMS, Nagpur. Required information including age, gender, major complains, other symptoms of the disease, duration of symptom onset before hospitalisation, records of close contact, were collected using standardised forms. Out of 50 cases, 13 cases were excluded, which included patients with age greater than 60 years (n=4), took discharge against medical advice (n=5) and who didn’t provide consent for the study (n=4). A total of 37 cases were further enrolled. Out of 37 cases, 17 cases with CSF unavailability and abnormal CSF findings/or having mixed infection were further excluded. Total 20 TBM cases were finally diagnosed [Table/Fig-1].
Study flow diagram for participant recruitment.
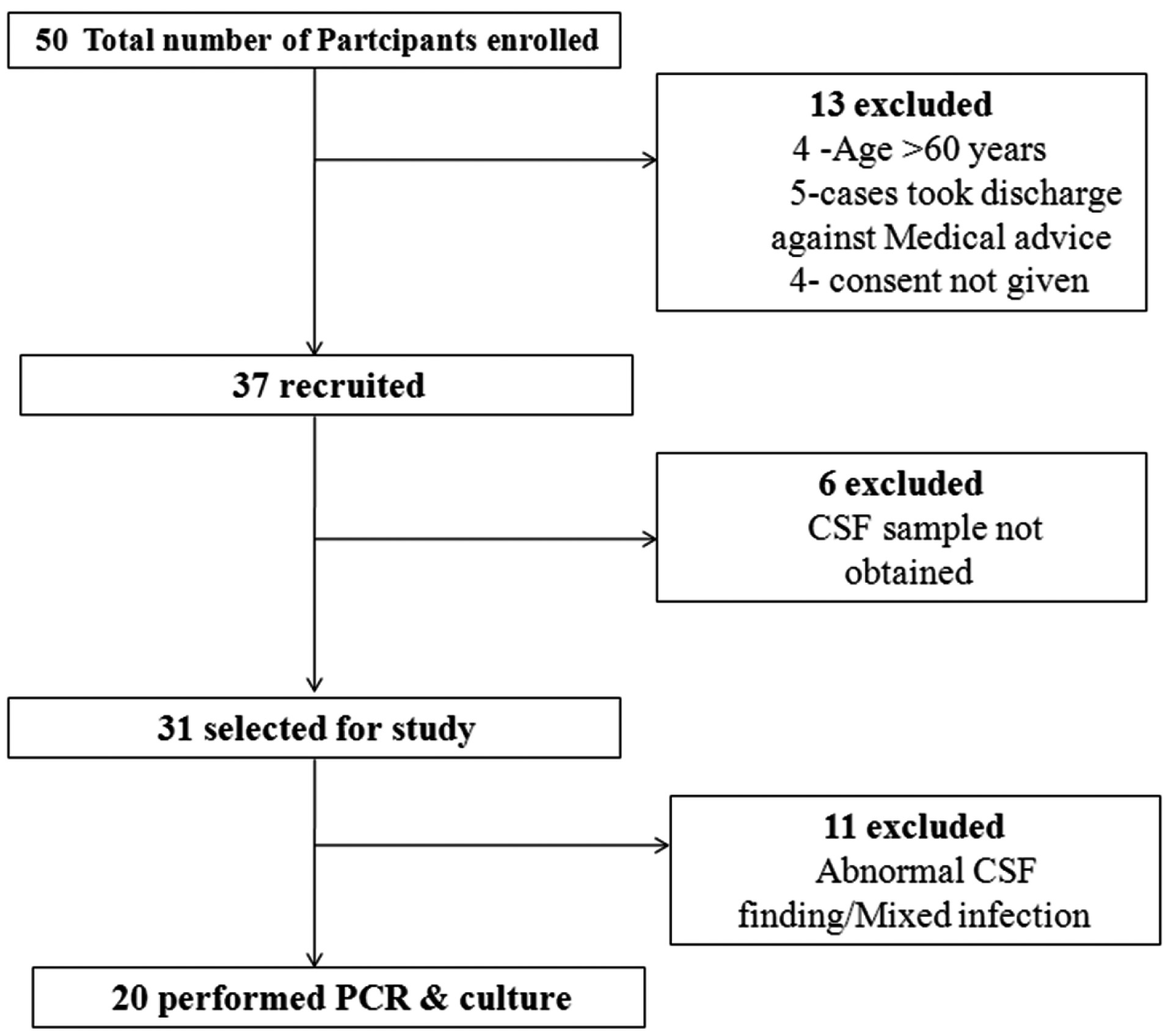
TBM diagnosis in recruited cases was based on an abnormality in Computerised Tomography (CT) and Magnetic Resonance Imaging (MRI) findings and clinical features suggestive of TBM infections. Clinical features for diagnosis included sub-acute or chronic fever with signs of meningeal irritation and CSF smear/or culture positive for Acid Fast Bacilli (AFB). CSF findings in these patients included increased protein levels, decreased glucose levels (CSF/blood glucose ratio, ≤0.5). All the patients with more than one week of symptoms initiation were evaluated. Patients with abnormal CSF findings and positive for other infections along with TBM were excluded from the study. CSF and peripheral blood samples were obtained from almost all TBM patients before initiation of Anti- Tuber closis Treatment (ATT) and utilised for PCR and culture analysis.
The 20 recruited cases were further categorised based on the severity of TBM at the time of admission in three stages as described elsewhere [13]. In brief, TBM patients were categorized on the basis of BMRC contemporary clinical criteria for the severity of TBM [14]. Glasgow Coma Score (GCS) and neurological defect were taken as primary criteria for grading. A low GCS corresponds to severity of disease. Grade 1 included patients with a GCS of 15 with no focal neurology. Grade II included patient with a GCS of 14 to 11 with focal neurological deficits. Grade -III patient had GCS of 10 or less, with or without focal neurological deficits.
For analysis, 2-3 ml CSF was collected using standard lumbar puncture method under sterile condition. For peripheral blood collection, 5 ml venous blood was collected directly in sterile ethylenediamine tetraacetic acid (EDTA) vacutainer on the same day of CSF collection. Both collected samples were immediately refrigerated at 4oC until further use.
DNA Extraction from CSF and Peripheral Blood Sample
a) CSF
DNA was extracted from CSF samples by phenol chloroform extraction method as described earlier by Deshpande PS et al., [6]. CSF was centrifuged at high speed (12000 rpm) for 10 minutes and the pellet was dissolved in sterile Phosphate Buffered Saline (PBS). A 15 μl of 10% SDS and 3 μl of 10 mg/ml Proteinase K was added and incubated at 55°C. After incubation 100 μl of 5 M NaCl and 80 μl of CTAB/NaCl solution were added and incubated for 10 minutes at 65°C. Equal volumes of phenol/ (chloroform/isoamyl alcohol) was added in 1:1 ratio and the tube was centrifuged for 10 minute at 12,000 rpm. Aqueous supernatant was then transferred to a fresh tube and 0.7-0.8 ml of chloroform/isoamyl alcohol was added, mixed thoroughly and centrifuged for 10 min at 12,000 rpm. The supernatant was then transferred to a fresh tube, and 30 μl of sodium acetate and 0.6 volumes of isopropanol were added and kept in -20oC to precipitate the DNA for half an hour. The mixture was then centrifuged at 12,000 rpm for 15 minute to obtain pellet, the supernatant was discarded and 1 ml of 70% ethanol was added. The tube was centrifuged for 10 minute at high speed and again the supernatant was discarded and the pellet was air dried. The pellet was resuspended in 25 μl of TE buffer for further use in PCR.
b) Peripheral Blood
For DNA isolation from peripheral blood, Buffy coat was separated by gradient centrifugation using HISTOPAQUE – 1077 (St. Louis, Mo. SIGMA) described elsewhere [15]. In brief, 6 ml of PBS was thoroughly mixed with 2 ml of peripheral blood. An 8 ml of peripheral blood + PBS mixture was added gradually from the sides of the tube to 4 ml of histopaque and centrifuged at 2000 rpm for 10 minutes. The buffy coat thus obtained was transferred to a fresh tube and equal volume of PBS was added and centrifuged again at 2000 rpm for 10 minute. Supernatant obtained was discarded and pellet, thus obtained was used for DNA isolation by phenol chloroform extraction method described earlier for CSF.
Conventional PCR
Amplification of PCR products was carried out by in-house protocol using a specific pair of primers designed to amplify an insertion sequence IS6110 in the MTB complex having expected band size of 123-bp [6,16]. The sequences of forward and reverse primers were 5’-CCT GCG AGC GTA GGC GTC GG-3’ and 5’-CTC GTC CAG CGC CGC TTC GG-3’, respectively. Amplification was carried out in a thermal cycler (Applied Biosystems, Foster city, Calif., USA), involving 40 cycles of denaturation at 94°C for 1 minutes, annealing of primers at 68°C for 1 minutes, and primer extension at 72°C for 10 minutes. The positive control included the DNA of MTB H37Rv strain provided by Colorado State University, Fort Collins, USA, (Contract No 1-A1-40091). Amplified products were detected by electrophoresis on 2% agarose gel and visualized on a UV gel documentation system (Bio- Rad Laboratories, CA, USA). The preparation of master mix was carried out in a separate a PCR/UV workstation. To prevent cross-contamination, different sets of pipettes and distinct work areas were used for DNA template preparation, PCR mixture preparation, DNA amplification and gel analysis. Moreover, one positive and one negative control were included with every set of samples used during DNA extraction and PCR.
Statistical Analysis
Clinical characteristics were summarised in terms of percentages for categorical variables i.e., age groups, gender, signs and symptoms. All statistical analysis was carried out using MedCalc software version 11.6.
Results
Out of 50 enrolled cases, 20 participants were finally selected for the study. The distribution of cases based on age, gender and clinical characteristics are summarised in [Table/Fig-2]. Out of 20 participants, there were 8 males and 12 females with a median age of 33.5 years (range-18-60 years). There were a number of cases with neck stiffness (70%), headache (70%) and fever (80%) representing classical manifestations of TBM infection. A total of 9 cases represented abnormality either in brain CT (25%) or MRI (20%). Almost 60% cases showed improvement post treatment, while 30% showed neurological abnormality post hospital discharge. Two cases (10%) were expired during their hospital stay.
Baseline characteristics of study populations.
| Sr. No. | Characteristics | Total (n= 20) (%) |
|---|
| 1. | Age |
| <18 | 2 (10) |
| 18 to 40 | 8 (40) |
| >40 | 10 (50) |
| 2. | Gender | |
| Male | 8(40) |
| Female | 12 (60) |
| 3. | Clinical symptoms |
| Fever | 16 (80) |
| Headache | 14 (70) |
| Limb weakness | 6 (30) |
| Neck stiffness | 14 (70) |
| 4. | Imaging |
| CT-scan | 5(25) |
| MRI | 4(20) |
| 5. | Clinical Outcome |
| Improved | 12(60) |
| Not improved | 6(30) |
| Expired | 2 (10) |
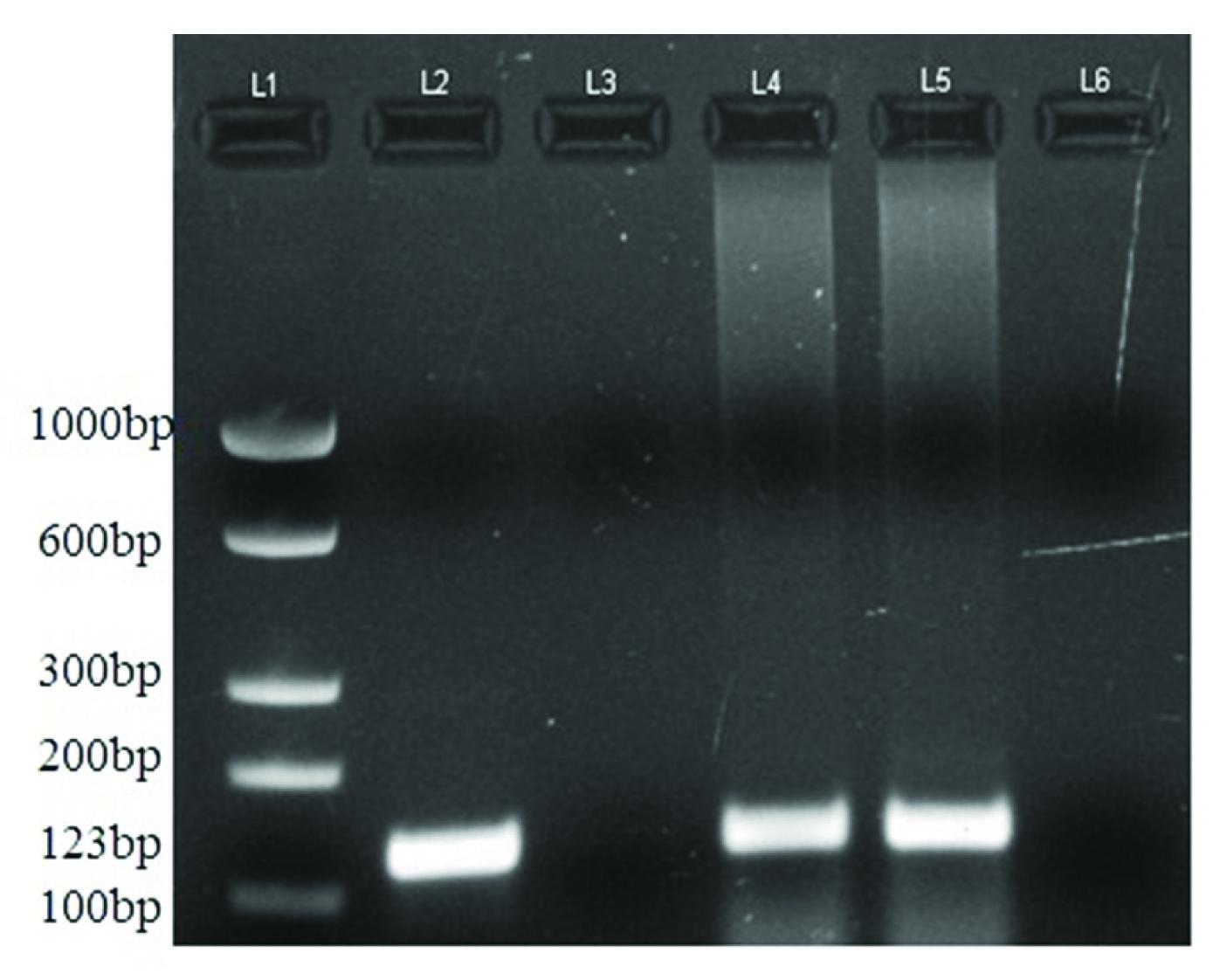
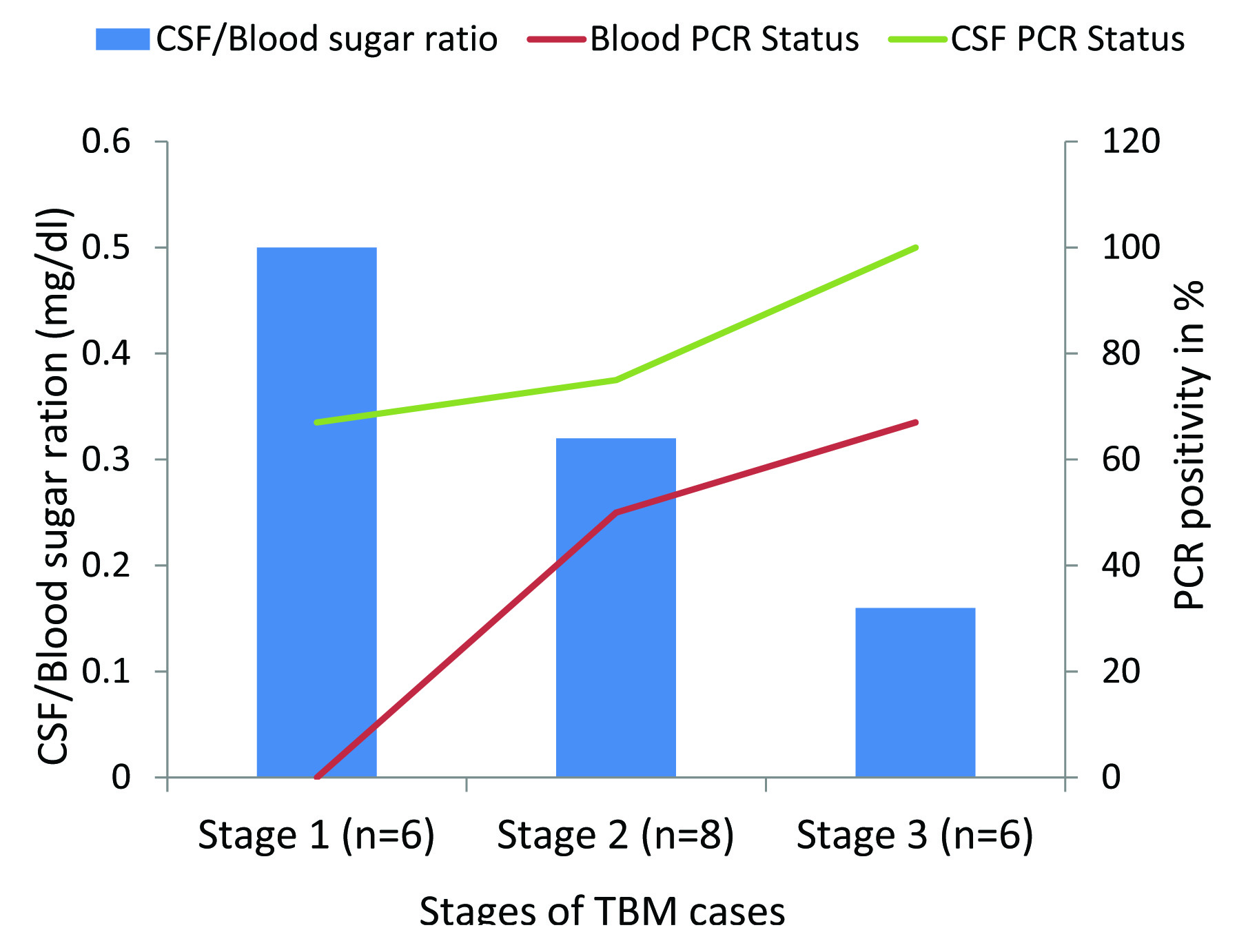
[Table/Fig-3] shows application of 123bp product of MTB by PCR in peripheral blood and CSF samples of TBM patients. PCR products were analysed by electrophoresis on 2% agarose gel. [Table/Fig-4] shows comparative results of CSF and peripheral blood PCR along with culture results and CSF/Blood sugar ratio in all the three TBM stages. Peripheral blood PCR yielded positivity about 50% and 67% in stage 2 and 3 TBM infection compared to CSF where PCR positivity was around 75% and 100%. No positivity of peripheral blood PCR was found in stage 1 TBM infection, compared to CSF, which showed higher positivity rates of 67%. Culture positivity rates were around 17%, 38% and 50% in different TBM stages which were less than PCR positivity reported in peripheral blood and CSF. Mean CSF/blood sugar ratio was around 0.50 (±0. 36) in stage 1, 0.32 (±0.08) in stage 2 and 0.16 (±0.11) in stage 3. Higher positivity PCR rates were associated with decrease sugar ratio in peripheral blood (50% and 67%) and CSF (75% and 100%) in stage 2 and 3 cases respectively as shown in [Table/Fig-5].
Amplification of the 123 bp product of M.tuberculosis by IS6110PCR in CSF and peripheral blood samples of TBM patients. L1: 100 bp ladder. L2: Positive control of Mycobacterium tuberculosis. L3 and L6: Negative control. L4: Patient’s peripheral blood sample. L5: Patient’s CSF sample.
Shows comparative results of CSF and peripheral blood PCR along with culture results and CSF/Blood sugar ratio in three TBM stages.
| Stages (n=20) | PCR Positivity | Culture positivity | CSF/Blood sugar ratio mg/dl (mean) |
|---|
| CSF | Blood |
|---|
| I (n=6) | 4 (67%) | 0 (0%) | 1 (17%) | 0.50 (±0.36) |
| II (n=8) | 6 (75%) | 4 (50%) | 3 (38%) | 0.32 (±0.08) |
| III (n=6) | 6 (100%) | 4 (67%) | 3 (50%) | 0.16 (±0.11) |
Comparative positivity of IS6110 PCR in CSF and peripheral blood samples with CSF/blood sugar ratio in three stages of TBM infection.
[Table/Fig-6] shows the comparative diagnostic utility of peripheral blood PCR assay in CSF PCR/culture positive and negative TBM groups. Overall sensitivity and specificity of peripheral blood PCR in CSF PCR/culture positive group was around 50% and 100% respectively. The diagnostic utility of peripheral blood PCR was considerably reduced in CSF PCR/culture negative groups with sensitivity & specificity ranging from 24.72-75.29% & 40.23-100%. The positive predictive value of blood PCR was 100% in TBM cases positive for both CSF culture and PCR, which decreased to 62.91% when both were negative.
Comparative diagnostic utility of peripheral blood PCR assay in TBM cases with CSF PCR/ culture positive and negative results.
| Peripheral blood PCR (n=20) | CSF PCR/ Culture |
|---|
| *Positive (n=16) | #Negative (n=4) |
|---|
| Positive (n=8) | 8 | 0 |
| Negative (n=12) | 8 | 4 |
| Sensitivity | 50.00% | 24.71% to 75.29% |
| Specificity | 100.00% | 40.23% to 100.00% |
| Positive Likelihood Ratio | ∞ | ∞ |
| Negative Likelihood Ratio | 0.5 | 0.31 to 0.82 |
| Disease prevalence | 80.00% | 56.33% to 94.14% |
| Positive Predictive Value | 100.00% | 62.91% to 100.00% |
| Negative Predictive Value | 33.33% | 10.13% to 65.05% |
*Represent total cases of CSF positive/Culture positive+CSF positive/Culture negative + CSF negative/ Culture positive
#Represent total cases of CSF negative/Culture negative
∞ indicates large and often conclusive increase in the likelihood of disease
Discussion
The objective of the current study was to investigate diagnostic utility of in house IS6110 PCR assay in peripheral blood and CSF samples for TBM infection. Both peripheral blood and CSF samples were collected from recruited TBM patients and were analysed using IS6110 PCR assays for MTB detection. Results suggested that although the molecular diagnosis using CSF remains a method of choice, in cases with TBM severity, peripheral blood can be used as an alternate source for TBM diagnosis particularly in case of CSF unavailability.
Despite the recent advances, clinical diagnosis of TBM infection has remained challenging due to existing limitation in current diagnostic tests. Conventional diagnostic methods such as direct smear microscopy and MTB isolation in bacteriological culture are inadequate for early diagnosis, owing to the poor sensitivity and high turn around (4–8 weeks) for cultures [17]. Detection of antibodies and antigen in the CSF to diagnose TBM is rapid method, but has shown suboptimal sensitivity & specificity for accurate diagnosis due to paucilbacillary nature along with problems of cross reactivity [17,18].
Molecular detection of TBM based on Mycobacterium specific insertion sequences has emerged as a promising tool for diagnosis owing to its rapidity and high sensitivity. IS6110 is a long 1191 bp repetitive insertion sequence that is usually present 6-20 times in the MTB complex genome than other repetitive sequence [3]. Many reports, including those from our own laboratory have demonstrated the value of Mycobacterium specific IS6110 PCR assay for diagnosis of extrapulmonary TB, including TB meningitis compared to CSF microscopy and culture [3,5,6,19]. In recent times newer molecular techniques like Loop Mediated Isothermal Amplification (LAMP) which requires much less infrastructure than conventional PCR have been investigated. Such assays are associated with better sensitivity and specificity, making it an attractive tool for TBM diagnosis in low middle income countries [20,21]. However, major limitation associated with PCR based assay for EPTB diagnosis includes the requirement of samples from extra pulmonary sites. Most of conventional and new methods for TBM diagnosis are dependent on the collection of CSF by spinal tap. However, large volumes of CSF may be required for confirmatory diagnosis since the sensitivity of both culture and PCR may vary depending upon initial bacterial load and volume collected [7]. The repeated collection of CSF may pose a disproportionate amount of stress and pain to the patient due to an invasive procedure and may not necessarily yield the same volume every time [13]. These problems warrant less perilous and more accessible clinical sample for MTB diagnosis.
Studies by investigators have shown that TBM begins with a primary respiratory infection followed by early haematogenous dissemination to CNS. During the progression of disease, bacteria may disseminate to local lymph nodes and bloodstream, which further spreads throughout the systemic circulatory system eventually crossing Blood Brain Barrier (BBB) and colonizing brain [22,23]. Similarly, the presence of MTB in peripheral blood has been demonstrated occasionally in the past by animal inoculation and culture. These findings were important to establish the accepted concept that the bacterium is the natural way that tuberculosis (TB) is spread in disseminated infections [24]. The resultant pathogenesis of MTB in TBM infection indicate us that peripheral blood may be used as a good alternative clinical specimen in TBM patients for MTB diagnosis using PCR.
Peripheral blood based PCR have been used in earlier studies for diagnosis of EPTB infection (pleural TB) with sensitivity of about 60%, which is superior to current sensitivity of CSF microscopy and culture [12]. Through our comprehensive literature search in Pubmed and other database, we found no studies that report utility of peripheral blood in TBM diagnosis using molecular assays. Our study is therefore first to report diagnostic utility of IS6110 PCR assays in peripheral blood for TBM cases. Although through our studies, we found higher positivity of conventional CSF based PCR for TBM diagnosis in all three TBM stages, increased positivity rates of peripheral blood PCR was associated with severity of infection, from stage 2 (50%) to stage 3 (67%) which was higher than liquid culture (38% and 50%) in both stages. The results are indicative that in cases with TBM severity, peripheral blood can be used as an alternate tool for diagnosis in case of CSF unavailability. The higher positivity of peripheral blood PCR in stage 3 TBM cases is indicative of higher mycobacterial dissemination in peripheral blood. Although no reports are available that supports our formulated hypothesis, since the available data on such studies are lacking.
Another important parameter which correlated with both CSF and peripheral blood based PCR assay was CSF/Blood sugar ratio. CSF/blood sugar ratio has been regarded as one of key inclusion parameters for clinical diagnosis of TBM infection and its differentiation from non mycobacterial CNS infection [25,26]. We included CSF/blood sugar ratio in our analysis as a correlation of infection and severity. Mean CSF/blood sugar ratio in three TBM stages was found to be 0.50 (±0.36), 0.32 (±0.08), 0.16 (±0.11). In our study, stage 3 TBM cases with less CSF/blood sugar ratio (0.16 ± 0.11) showed higher positivity rates for PCR assays (67%) indicating a high probability of MTB detection in peripheral blood. The above combitorial test approach can be helpful in confirming TBM diagnosis in probable cases having CSF smear/culture negativity for MTB, thereby avoiding misdiagnosis. Another advantage of peripheral blood based PCR assay is sample volume, since 5-10 ml peripheral blood can be collected repeatedly compared to CSF and therefore can be used as good alternative in follow up analysis of TBM cases, where CSF volume is less or not available for analysis.
Although no studies are available for molecular diagnosis of TBM in peripheral blood, some investigators have shown peripheral blood to be a useful adjunct for the TBM diagnosis along with CSF, using T cell based assays. A recent study by Lu D et al., evaluated the diagnostic utility of peripheral blood T-SPOT. TB and CSF interferon-γ detection methods in TBM cases. When both T-SPOT.TB and CSF-IFN-γ results were positive, the specificity and positive predictive value of TBM diagnosis reached 100%, thereby indicating the combined use of T-SPOT.TB and CSF IFN-γ could improve the diagnostic efficiency of TBM [27]. In another study, Kim SH et al., showed ELISPOT assays using peripheral mononuclear cells and CSF mononuclear cells are useful adjuncts to the current tests for diagnosing TBM, particularly when used in combination with the assessment of adenosine deaminase level in CSF [28]. Peripheral blood culture has been reportedly used and yields results in 50–90% of patients with bacterial meningitis [20]. A study by Fuglsang-Damgaard D et al., suggest on utility of positive peripheral blood cultures for diagnosis of bacterial meningitis in cases with negative CSF culture [29]. Although information on utility of culture and molecular assays using peripheral blood for TBM diagnosis is sparse, our study provides a base for future investigation to be carried out on this aspect. Probably further investigation deciphering TBM pathogenesis using animal models may help us to bolster our understanding on the diagnostic utility of peripheral blood in the diagnosis of TBM infection in future.
Limitation
Our study is associated with major limitation which includes low sample size. Although with this low sample number we got 100% specificity, but only 50% sensitivity was found in peripheral blood based PCR assay. Since, studies were carried out at pilot scale for screening and comparative analysis, we recruited limited cases for ethical reasons. However, based on results obtained we plan to carry out study in larger cohorts for better validation to establish the role of peripheral blood based PCR for diagnosis of TBM infection.
Conclusion
Our results suggest, although molecular diagnosis of TBM infection in CSF remains the method of choice, peripheral blood based PCR can be used as a good alternative to CSF in case of TBM severity where the repeated CSF collection may be needed. However, study demands further validation in large cohorts to justify the present hypothesis.
*Represent total cases of CSF positive/Culture positive+CSF positive/Culture negative + CSF negative/ Culture positive
#Represent total cases of CSF negative/Culture negative
∞ indicates large and often conclusive increase in the likelihood of disease