Cardiac Amyloidosis, An Infiltrative Heart Disease Presenting as Arrhythmia-A Case Report
B Magesh1, Deepak Kadeli2, Sunil Bohra3, V Krishnaprasath4, R Keshava5
1 Junior Interventional Cardiologist, Department of Cardiology, Fortis Hospital, Bengaluru, Karnataka, India.
2 DNB Cardiology Resident, Department of Cardiology, Fortis Hospital, Bengaluru, Karnataka, India.
3 Non Invasive Cardiologist, Department of Cardiology, Fortis Hospital, Bengaluru, Karnataka, India.
4 DNB Cardiology Resident, Department of Cardiology, Fortis Hospital, Bengaluru, Karnataka, India.
5 Consultant Interventional Cardiologist, Department of Cardiology, Fortis Hospital, Bengaluru, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Deepak Kadeli, Fortis Hospital, Cunningham Road, Bengaluru- 52, Karnataka, India.
E-mail: kadelideepak@gmail.com
Cardiac amyloidosis is a manifestation of amyloidosis which is a multisystem disorder. This is difficult to diagnose, rare disease which eventually leads to the mortality. Diagnosis requires a high index of clinical suspicion along with echocardiographic clues like, diastolic dysfunction, bi-atrial enlargement and ventricular thickening. Treatment is mainly supportive with disappointing outcomes. We present a case of systemic amyloidosis with negative congo red staining, presenting with predominantly cardiac features.
Bi-atrial enlargement, Congo red staining, Macroglossia, Ventricular thickening
Case Report
A 63-year-old male presented with the history of palpitations. He had been having exertional breathlessness since one week. He had bilateral pedal oedema. Electrocardiogram showed Atrial Fibrillation (AF) with fast ventricular rate. He was given oral amidarone after which the AF reverted to sinus rhythm. The patient also had hoarseness of voice and difficulty in swallowing since past one month and on examination was found to have macroglossia.
Coronary angiogram showed normal coronaries. He was started on diuretics and antiplatelets.
Echocardiography showed dilated Left Atrium (LA), concentric Left Ventricular Hypertrophy (LVH) with changes suggestive of infiltrative disease of the myocardium [Table/Fig-1].
Arrow shows speckled granular appearance in the interventricular septum.
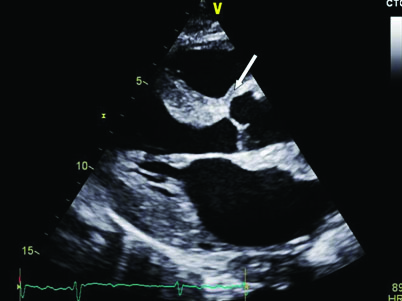
In view of infiltrative changes of myocardium and macroglossia a systemic infiltrative disease was suspected. Biopsy of the tongue revealed hyperplasic acini in the subepithelium.
Congo red stain for amyloid was inconclusive. The patient did not consent for abdominal pad biopsy. A cardiac MRI was done which suggested morphology of amyloidosis [Table/Fig-2].
MRI shows infiltrative deposits in the myocardium suggestive of cardiac amyloidosis.
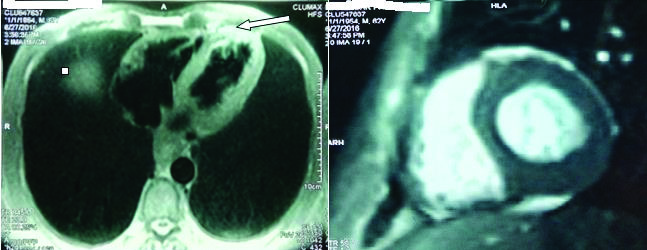
Bone marrow biopsy negative for amyloidosis but showed increased plasma cells (>20%). Urine was positive for Bence Jones proteins. Serum protein electrophoresis revealed an elevated alpha 2 globulin fraction. A diagnosis of plasma cell dyscrasia with systemic amyloidosis was made.
A diagnosis of systemic amyloidosis with predominant cardiac involvement was done. The patient’s family was counseled regarding the nature of disease and its progression and available treatment options. He was referred to a haemato-oncologist where he underwent chemotherapy with bortezomib, lenalidomide and dexamethosone.
Patient had two cycles of chemotherapy when he presented to emergency room with abdominal bloating which was diagnosed as megacolon. Patient was managed conservatively with decompression and improved. The patient is on regular follow up with continued chemotherapy.
Discussion
Amyloidosis patients usually present with non specific symptoms like generalized weakness, weight loss, palpitations and syncopal attacks. Specific symptoms may depend on the organ involved. In this case the organs predominantly involved were tongue, pharyngeal and larynx muscles, leading to macroglossia causing difficulty in swallowing and hoarseness of voice. The patient also had cardiac amyloidosis causing Atrial Fibrillation and features of diastolic failure.
Some of the case reports on cardiac amyloidosis comparing to the current case are described in [Table/Fig-3] [1-6].
Some of the case reports on cardiac amyloidosis comparing to the current case are described.
| Case Report | Presentation | Echo Findings | Cardiac MRI | Biopsy | Isolated Cardiac/Systemic Amyloidosis |
|---|
| Auoda A et al., | First degree AV block | Biatrial enlargement restrictive filling physiology | Not done | Endomyocardial biopsy positiveAbdominal fat pad negativeBone marrow biopsy positive | Systemic amyloidosis |
| Gilowski W et al., | Heart Failure | Biatrial enlargement restrictive filling physiology | Delayed gadolinium enhancement and an increased signal in T2 Dependent sequences | Gingival biopsy positiveAbdominal fat pad negativeEndomyocardial biopsy not done | Systemic amyloidosis |
| Khalid U et al., | Heart Failure | Biatrial enlargement restrictive filling physiology | delayed gadolinium enhancement | Myocardial biopsy positiveAbdominal fat pad negative | Isolated Cardiac amyloidosis |
| Mohan B et al., | Heart failure | Biatrial enlargement restrictive filling physiology | Not done | Abdominal fat pad negativeEndoscopic duodenal biopsy positiveEndomyocardial biopsy not done | Systemic amyloidosis |
| Nisha AG et al., | Polymorphic VT | Biatrial enlargement restrictive filling physiology | Not done | Abdominal fat pad negative Endomyocardial biopsy positive | Cardiac amyloidosis |
| Triantafyllou AI et al., | Heart failure | Biatrial enlargement restrictive filling physiology | Delayed gadolinium enhancement | Subcutaneous fat pad biopsy positive Bone marrow biopsy positiveEndomyocardial biopsy not done | Systemic amyloidosis |
| Present case | Atrial fibrillation | Biatrial enlargement restrictive filling physiology | Delayed gadolinium enhancement | Tongue biopsy negativeAbdominal fat pad not doneEndomyocardial biopsy not done | Systemic amyloidosis |
Biopsy from tissue specimen for examination of amyloid deposits is the gold standard for diagnosis, any source suspected of containing the abnormal protein may be used [7] abdominal fat-pad biopsy is the most preferred site [8,9] but may not always yield positive results. As seen in [Table/Fig-3], many cases do not yield amyloid positive biopsies in spite of taking samples from multiple sites. The amyloid protein can be visualized as salmon pink coloured amorphous substance seen extracellularly. When placed under polarized light the amyloid proteins have an apple-green birefringence which is considered pathognomonic for amyloid fibril deposits. Finally, immunohistochemical staining can be used to confirm for further confirmation.
Amyloidosis can present with very wide clinical spectrum but cardiac involvement is commonly seen and is sometimes the only presenting feature [10].
Cardiac amyloidosis presents as restrictive cardiomyopathy characterized by progressive diastolic and subsequently systolic biventricular dysfunction and arrhythmia just as in our case. They present with increased troponins which may lead to misdiagnosis. Increase in troponin levels is a marker of poor prognosis but the mechanism remains unclear.
Echocardiography plays an important role in diagnosis and under experienced hands can show several features that can be suggestive of cardiac amyloidosis [11], but these features are commonly present in later stages of the disease. Whenever there is high index of clinical suspicion, an ECHO showing a speckled or granular myocardial can be characterized as amyloidosis. Interatrial septum thickening is very specific for amyloid in the later stage of the disease, with 100% specificity [12]. Diastolic dysfunction is the hallmark and majority of patients show a restrictive pattern on mitral inflow assessment [13]. Advanced echocardiographic techniques with speckle tracking can be of great use in diagnosis [14].
Functional and morphological information can be better obtained by a cardiac Magnetic Resonance Imaging (MRI) with tissue characterization being an additional advantage. In clinical practice endomyocardial biopsy is rarely required though it is considered as gold standard for cardiac amyloidosis.
Treatment may be usually supportive; some therapies (chemotherapy) that suppress production of amyloid fibril precursor protein have been tried with varying results especially in AL amyloid. Heart failure therapy must be instituted cautiously as it may be of limited benefit or even be detrimental due to low blood pressure and arrhythmias. There is no evidence that pacemakers and other devices will benefit [15].
Conclusion
Although the initial presentation with classic echocardiographic, and cardiac MRI findings was suggestive of amyloidosis in our patient, negative biopsy from tongue, bone marrow made the diagnosis difficult. Endomyocardial biopsy being invasive procedure cannot always be done. Secondary causes of amyloidosis should always be sought after as some cases maybe amenable to treatment.
[1]. Aouda A, Toyozaki T, Saito T, Yorimitsu K, Miyazaki A, Deguchi F, A case of primary cardiac amyloidosis with amyloid A proteinKokyu To Junkan 1993 41(1):89-92. [Google Scholar]
[2]. Gilowski W, Stryjewski P, Gilowska M, Liszniański P, Nowak J, The rapid progress of heart failure due to systemic amyloidosis with cardiac involvement-case reportPrzegl Lek 2015 72(11):697-700. [Google Scholar]
[3]. Khalid U, Awar O, Verstovsek G, Cheong B, Yellapragada SV, Jneid H, Case report: Isolated cardiac amyloidosis: an enigma unraveledMethodist Debakey Cardiovasc J 2015 11(1):53-58. [Google Scholar]
[4]. Mohan B, Chhabra ST, Tandon R, Gupta NK, Aslam N, Sood NK, Cardiac amyloidosis – Two case reports with variable presentationJAPI 2011 59:263-66. [Google Scholar]
[5]. Nisha AG, Grant VC, Oscar HC, Cardiac amyloidosis with prolonged QTc and polymorphic VTTexas Heart Institute Journal 2013 40:193-95. [Google Scholar]
[6]. Triantafyllou AI, Kapelakis IE, Triantafyllou EA, Lampropoulos KM, Manolis AS, Cardiac amyloidosis: Mini review and a case reportInternational Journal of Cardiology and Lipidology Research 2015 2:14-19. [Google Scholar]
[7]. Rocken C, Sletten K, Amyloid in surgical pathologyVirchows Arch 2003 443(1):3-16. [Google Scholar]
[8]. Bardarov S, Michael CW, Pu RT, Pang Y, Computer assisted image analysis of amyloid deposits in abdominal fat pad aspiration biopsiesDiagn Cytopathol 2009 37(1):30-35. [Google Scholar]
[9]. Halloush R, Lavrovskaya E, Mody D, Lager D, Truong L, Diagnosis and typing of systemic amyloidosis: The role of abdominal fat pad fine needle aspiration biopsyCytojournal 2010 15(6):24 [Google Scholar]
[10]. Cristina QC, Jenna LK, Rodney HF, Cardiac amyloidosisCirculation 2012 126:178-82. [Google Scholar]
[11]. Rodney FH, Diagnosis and management of the cardiac amyloidosisCirculation 2005 112:2047-60. [Google Scholar]
[12]. Selvanayagam JB, Hawkins PN, Paul B, Evaluation and management of the cardiac amyloidosisJ Am Coll Cardiol 2007 50:2101-10. [Google Scholar]
[13]. Ruberg FL, Berk JL, Transthyretin (TTR) cardiac amyloidosisCirculation 2012 126(10):1286-300. [Google Scholar]
[14]. Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zacà V, Speckle tracking and myocardial functionJ Ultrasound Med 2011 30:71-83. [Google Scholar]
[15]. Seldin DC, Berk JL, Sam F, Sanchorawala V, Amyloidotic cardiomyopathy: Multidisciplinary approach to diagnosis and treatmentHeart Fail Clin 2011 7(3):385-93. [Google Scholar]