Osteosarcoma Arising in Carcinosarcoma De Novo Parotid Gland in a Young Man: An Unusual Case with Review of Literature
Vidya Jha1, Sachin Kolte2, Surbhi Goyal3, Ashish Kumar Mandal4
1 Senior Resident, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India.
2 Associate Professor, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India.
3 Assistant Professor, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India.
4 Director Professor, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Surbhi Goyal, Assistant Professor, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi-110029, India.
E-mail: dr.surbhi4you@gmail.com
Carcinosarcoma of the parotid gland, a true malignant mixed tumour is extremely rare. It may occur in a pre-existing pleomorphic adenoma or arise de novo. We report a case of carcinosarcoma de novo harbouring an osteosarcomatous element in a 35-year-old man along with review of the reported cases. Excision was done and histopathologic examination confirmed the diagnosis. Long term follow up has been recommended for these tumours owing to their high propensity of recurrence and metastasis. Our case discusses the importance of histopathology and limitation of preoperative imaging in the diagnosis of such an aggressive neoplasm; emphasizing the fact that possibility of carcinosarcoma should be kept in mind while dealing with salivary gland lesions even at a younger age.
Malignant mixed tumour, Neoplasm, Salivary gland
Case Report
A 35-year-old man presented with a swelling in the parotid region, left side which suddenly increased in size over a period of two months. There was no complaint of dysphagia, otalgia, hoarseness, weight loss or facial weakness. Past history was not significant.
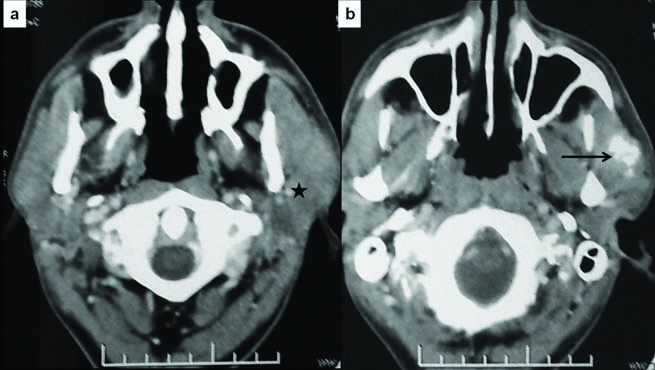
On examination, a firm, immobile and non-tender swelling measuring 4x3x2 cm, was palpable in the left parotid region. There were no signs of facial weakness or palpable lymphadenopathy. A preoperative Contrast Enhanced Computed Tomography (CECT) scan revealed a solitary, poorly defined, heterogeneously enhancing lesion (2.6x2.5x2.1 cm) arising from the anterior aspect of superficial lobe of left parotid gland [Table/Fig-1]. Coarse nodular calcification was seen within the lesion. Possibility of primary parotid tumour was suggested. Fine needle aspiration showed few atypical cells and was inconclusive for malignancy. The patient underwent total radical parotidectomy with excision of the upper trunk of facial nerve as it was found to be involved by the tumour peroperatively.
a) Axial CECT images of neck show ill-defined heterogeneously enhancing mass lesion in the left parotid, predominantly involving the superficial lobe (asterisk); b) Coarse chunky calcification and ossification in the anteroinferior component of the lesion (arrow), invading the masseter muscle.
A total parotidectomy specimen was received. Superficial and deep lobe measured 4x4x2 cm and 6x5x1.5 cm respectively. A gray white, firm to hard tumour could be identified in the superficial lobe measuring 3.5x3.4x1.8 cm. On cutting, the tumour was gritty.
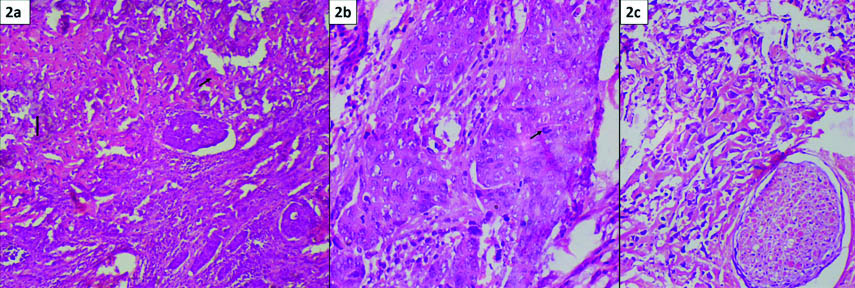
Microscopic examination revealed, a poorly circumscribed tumour comprising of two distinct components intermingling with each other. The epithelial component was seen in form of sheets, trabeculae and cords. Tumour cells were round to polygonal with abundant eosinophilic cytoplasm and prominent nucleoli [Table/Fig-2]. Other element comprised of malignant spindle cells arranged in fascicles and sheets, showing marked pleomorphism and nuclear atypia. At places, these cells were entrapped within pink lacy osteoid [Table/Fig-3]. Focal areas of calcification and necrosis were seen. Atypical mitoses were identified in both the components (1-2/hpf). Perineural invasion was noted [Table/Fig-2]. On immunohistochemistry, the epithelial cells were positive for epithelial membrane antigen (EMA), cytokeratin and MUC-1 [Table/Fig-4]. They were negative for vimentin, p63, Smooth Muscle Actin (SMA) and S-100. In contrast, the spindle cells were positive for vimentin and negative for EMA, cytokeratin, p63, SMA, S-100 and desmin [Table/Fig-4]. One lymph node was received which showed tumour metastasis. Entire specimen was processed to look for any component of benign mixed tumour. After extensive sampling no evidence of pre-existing pleomorphic adenoma was found. Thus, a final diagnosis of carcinosarcoma de novo of the parotid gland was rendered. Patient was given adjuvant radiotherapy and he is healthy with no evidence of recurrence or metastasis 12 months after follow up.
a) Section shows a biphasic tumour comprising of poorly differentiated adenocarcinoma along with sarcomatous areas. Foci of osteosarcomatous differentiation (short arrow) with calcification (long arrow) are seen. (H&E 20X); b) Higher magnification shows malignant epithelial cells arranged in sheets and nests. These cells are polygonal, have moderate eosinophilic cytoplasm and pleomorphic vesicular nuclei with conspicuous nucleoli. Few atypical mitoses are seen (arrow) (H&E 40X); c) Section shows perineural invasion. (H&E 20X).
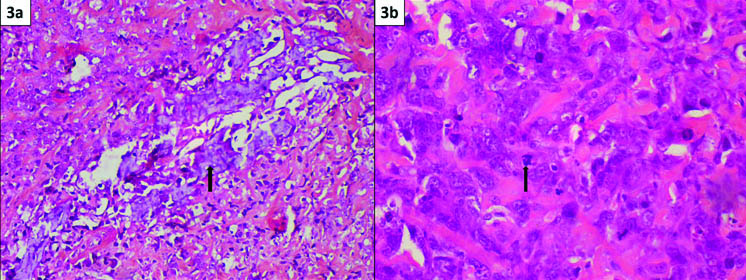
a) Osteosarcomatous element with foci of calcification (arrow) (H&E 20X); b) Higher magnification shows round to elongated tumour cells entrapped within osteoid. These cells are pleomorphic having hyperchromatic nuclei and prominent nucleoli. Few atypical mitoses are seen (arrow) (H&E 40X).
a) Immunohistochemistry- Tumour cells show membranous positivity for epithelial membrane antigen (EMA 20X); b) Tumour cells are positive for vimentin in osteosarcomatous areas (vimentin 40X).

Discussion
First described in 1951, carcinosarcoma (true malignant mixed tumour) of the parotid gland is an extremely rare tumour, accounting for 0.4% of malignant salivary gland neoplasms [1-3]. It is a biphasic tumour comprising of carcinoma along with sarcomatous elements. Usual epithelial components include squamous cell carcinoma, adenocarcinoma and undifferentiated carcinoma while chondrosarcoma and osteosarcoma usually represent the sarcomas. Rarely fibrosarcoma, leiomyosarcoma, or liposarcoma can be seen [1-3].
Malignant Mixed Tumour (MMT) of the salivary glands is of three histologic subtypes. Carcinoma ex pleomorphic adenoma is the most common, accounting for approximately 50% of cases. Metastasizing mixed tumour is the most infrequent subtype. Carcinosarcoma (true MMT) has been described in gastrointestinal tract, respiratory tract, urogenital system and breast. In the salivary glands, it most frequently occurs in the parotid (69.5%), followed by the submandibular gland (17.4%). It affects a wide age range with a predilection towards seventh decade [4].
Sonography followed by CECT/MRI are employed for salivary gland lesions. Imaging is nonspecific and unable to differentiate malignant from benign lesions, though can delineate exact tumour site and detect local invasion [4].
We found poorly differentiated adenocarcinoma intermingling with osteosarcoma in our case. To the best of our knowledge, only nine cases of carcinosarcoma de novo harbouring an osteosarcomatous element have been reported [Table/Fig-5] [5-13]. We report first such case involving the parotid gland in a young man of 35 years. In these reported cases, male:female ratio was 2:1 with a mean age of 65.2 years. Six cases occurred in the parotid gland, submandibular gland was involved in the remaining three. Adenocarcinoma and ductal carcinoma represented the carcinoma in all the cases. Amongst sarcomas, chondrosarcoma (five cases), fibrosarcoma and rhabdomyosarcoma (one case each) were found [5-13].
Carcinosarcoma of salivary glands with osteosarcomatous differentiation: A review of reported cases [5-13].
| Author | Age in years/Sex | Type of carcinoma | Type of sarcoma | Location |
|---|
| Garner SL et al., [5] | 57/F | Adenoc-arcinoma | Osteosarcoma, chondrosarcoma | Parotid gland |
| Yamashita T et al., [6] | 52/M | Poorly differentiated adenoc-arcinoma with squamous cell differentiation | Osteosarcoma. Chondrosarcoma, Fibrosarcoma | Subman-dibular gland |
| Bleiweiss IJ et al., [7] | 64/M | Ductal adenoc-arcinoma | Osteosarcoma | Subman-dibular gland |
| de la Torre M and Larsson E [8] | 83/F | Adenoc-arcinoma | Osteosarcoma, Chondrosarcoma | Parotid gland |
| Carson HJ et al., [9] | 51/F | Adenoc-arcinoma | Osteosarcoma | Parotid gland |
| Gogas J et al., [10] | 77/M | Ductal adenoca-rcinoma | Osteosarcoma. Chondrosarcoma, Rhabdomyosarcoma | Subma-ndibular gland |
| Sironi M et al., [11] | 77/M | Ductal adenoca-rcinoma, squamous cell carcinoma | Osteosarcoma | Parotid gland |
| Mardi K and Sharma J [12] | 59/M | Ductal adenoca-rcinoma | Osteosarcoma, Chondrosarcoma | Parotid gland |
| Jang S et al., [13] | 67/M | Adenoc-arcinoma | Osteosarcoma | Parotid gland |
| Present case | 35/M | Poorly differentiated adenoc-arcinoma | Osteosarcoma | Parotid gland |
Carcinosarcoma of the salivary gland is an aggressive tumour with an average survival of 3.6 years after treatment [14]. Local recurrence and metastasis via lymphatic and haematologic routes to regional sites are seen in 50% cases. Most common site of metastasis is lung followed by bone, liver and brain [14]. The biphasic nature of the tumour might pose a diagnostic challenge at metastatic sites.
Treatment consists of either surgery alone or followed by radiotherapy/chemotherapy. Surgery followed by radiotherapy has a significantly lower recurrence rate than excision alone. Benefits of chemotherapy are controversial [4].
There are two main hypotheses for the genesis of carcinosarcoma. Collision theory implies development of two independent elements which then intermingle [14]. Monoclonal hypothesis, more widely accepted, implies a common precursor in the form of dedifferentiated or pluripotent cells that undergo divergent differentiation [7].
The sarcomatous element tends to predominate over the carcinoma; therefore immunohistochemistry (EMA, cytokeratin) is recommended to distinguish carcinosarcoma from primary sarcoma. Coexistence of typical pleomorphic adenoma as well as immunohistochemical positivity for S-100 and SMA are suggestive of carcinoma ex pleomorphic adenoma [7,11,14]. In our case, neither of the components showed immunoreactivity for S-100 and SMA. This might be explained by the fact that denovo cases lack evidence of myoepithelial origin.
Conclusion
To conclude, it is very unusual to encounter carcinosarcoma de novo at a young age of 35 years. Preoperative diagnosis is difficult due to nonspecific clinicoradiological findings, warranting the need of histopathology for confirmation. It has a very poor prognosis with high chances of recurrence and metastases. Surgery along with long term follow up is mandatory in such patients.
[1]. Kirklin JW, McDonald JR, Harrington SW, Parotid tumours: histopathology, clinical behaviour and end resultsSurg Gynecol Obstet 1951 92:721-33. [Google Scholar]
[2]. Gnepp DR, Malignant mixed tumours of the salivary glands: a reviewPathol Annul 1993 28:279-28. [Google Scholar]
[3]. Taki H, Laver N, Quinto T, Carcinosarcoma de novo of the parotid gland: case reportHead Neck 2013 35:E161-63. [Google Scholar]
[4]. Staffieri C, Marioni G, Ferraro SM, Marino F, Staffieri A, Carcinosarcoma de novo of the parotid glandOral Surg Oral Med Oral Pathol Oral Radiol Endod 2007 104:e35-e40. [Google Scholar]
[5]. Garner SL, Robinson RA, Maves MD, Barnes CH, Salivary gland carcinosarcoma: true malignant mixed tumourAnn Otol Rhinol Laryngol 1989 98:611-14. [Google Scholar]
[6]. Yamashita T, Kameda N, Katayama K, Hiruta N, Nakada M, Takeda Y, True malignant mixed tumour of the submandibular glandActa Pathol Jpn 1990 40:137-42. [Google Scholar]
[7]. Bleiweiss IJ, Huvos AG, Lara J, Strong EW, Carcinosarcoma of the submandibular salivary gland: immunohistochemical findingsCancer 1992 69:2031-35. [Google Scholar]
[8]. de la Torre M, Larsson E, Fine-needle aspiration cytology of carcinosarcoma of the parotid gland: cytohistological and immunohistochemical findingsDiagn Cytopathol 1995 12:350-53. [Google Scholar]
[9]. Carson HJ, Tojo DP, Chow JM, Hammadeh R, Raslan WF, Carcinosarcoma of salivary glands with unusual stromal components: report of two cases and review of the literatureOral Surg Oral Med Oral Pathol Oral Radiol Endod 1995 79:738-46. [Google Scholar]
[10]. Gogas J, Markopoulos C, Karydakis V, Gogas G, Delladetsima J, Carcinosarcoma of the submandibular salivary glandEur J Surg Oncol 1999 25:333-35. [Google Scholar]
[11]. Sironi M, Isimbaldi G, Claren R, Delpiano C, Di Nuovo F, Spinelli M, Carcinosarcoma of the parotid gland: cytological, clinicopathological and immunohistochemical study of a casePathol Res Pract 2000 196:511-17. [Google Scholar]
[12]. Mardi K, Sharma J, True malignant mixed tumour (carcinosarcoma) of parotid gland: a case reportIndian J Pathol Microbiol 2004 47:64-66. [Google Scholar]
[13]. Jang S, Young Y, Han H, Parotid gland carcinosarcoma with osteosarcoma as a sarcomatous component: a case report with fine needle aspiration cytologic findingsKorean J Pathol 2011 45:412-16. [Google Scholar]
[14]. Stephen J, Batsakis JG, Luna MA, von der Heyden U, Byers RM, True malignant mixed tumours (carcinosarcoma) of salivary glandsOral Surg Oral Med Oral Pathol 1986 61:597-02. [Google Scholar]