Leiomyosarcoma of Renal Vein - A Rare Case Report
Sandeep Ojha1, Ramarao Nilkanthe2, Jyoti Valecha3, Farah Meenai4, Amit Haritwal5
1 Assistant Professor, Department of Pathology, Chirayu Medical College and Hospital, Bhopal, Madhya Pradesh, India.
2 Assistant Professor, Department of Pathology, Chirayu Medical College and Hospital, Bhopal, Madhya Pradesh, India.
3 Associate Professor, Department of Radiology, Chirayu Medical College and Hospital, Bhopal, Madhya Pradesh, India.
4 Associate Professor, Department of Pathology, Chirayu Medical College and Hospital, Bhopal, Madhya Pradesh, India.
5 Associate Professor, Department of Pathology, Chirayu Medical College and Hospital, Bhopal, Madhya Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sandeep Ojha, Assistant Professor, Department of Pathology, Chirayu Medical College, Bhaisakhedi, Bhopal Indore Highway, Bhopal - 462030, Madhya Pradesh, India.
E-mail: drsandy0582@gmail.com
Leiomyosarcomas (LMS) arising from vascular channel are rare and more often arise from inferior vena cava and pulmonary arteries. Primary renal vein LMS are even rarer and occur predominantly in females with peak in fifth and sixth decade. Preoperative diagnosis is difficult because these are rare tumours and present with symptoms and radiological findings similar to Renal Cell Carcinoma (RCC). We report a case of 55-year-old female who presented with abdominal discomfort with radiology showing a renal mass with features of RCC, radical nephrectomy was done and resected tumour showed an attachment to the wall of renal vein with morphology resembling LMS.
Female, Kidney neoplasms, Nephrectomy
Case Report
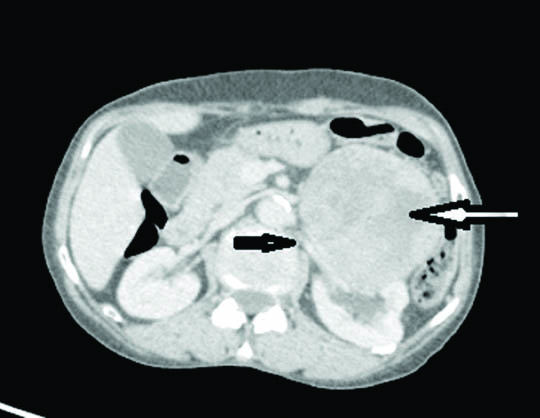
A 55-year-old female presented to outpatient department with complaints of left flank pain, vomiting and abdominal fullness. CT scan revealed a large mass at lower pole of left kidney which appeared to arise from renal parenchyma and was in close proximity with hilar structures. Renal vein appeared to be patent and tumour was seen anterior to the renal vein wall [Table/Fig-1]. No tumour emboli were seen in renal vein and no other lesions suggestive of distant metastasis were seen. Diagnosis of RCC was made as imaging findings suggested origin of tumour in kidney. Due to well circumscribed nature of the tumour favouring operability, radical nephrectomy was performed and sent for histopathological examination.
CT image showing a mass near hilum of kidney (white arrow) anterior to the renal vein (black arrow) but not infiltrating it.
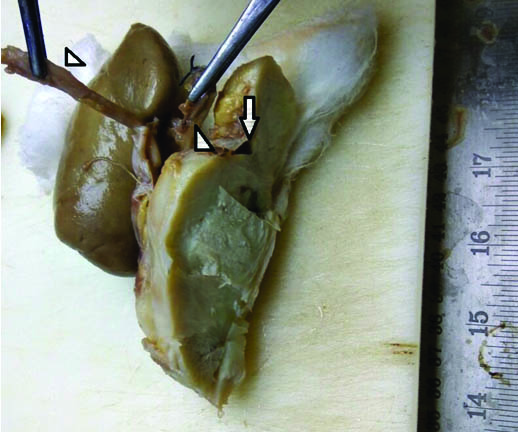
Gross specimen showed a mass near the hilum of left kidney. The mass was well circumscribed measuring 10x9x9 cm and appeared to arise from the wall of renal vein anteriorly [Table/Fig-2]. Lumen of the renal vein showed blood clot and did not show any invasion by tumour. Capsule of the kidney was stripped off with ease and kidney was completely free from the tumour mass. Renal artery and ureter were free from tumour. On serial slicing, tumour was gray white, fleshy with focal soft areas. No areas of hemorrhage or necrosis were seen.
Gross specimen showing tumour separate from kidney with unremarkable ureter and renal artery (arrow heads). Renal vein showed a tumour arising from anterior wall (arrow).
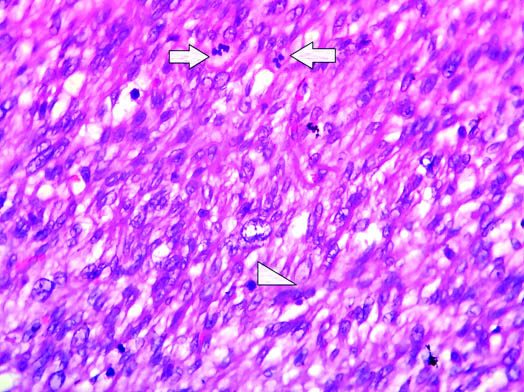
Microscopy showed a spindle cell neoplasm arising from the wall of renal vein showing fascicular pattern of growth with few bundles intersecting perpendicular to each other. Tumour cells showed moderate to severe atypia diffusely. Mitotic activity was >10/10 HPF in the highest proliferating areas [Table/Fig-3]. Areas of necrosis were not seen, however in view of presence of moderate to severe nuclear atypia diffusely and increased mitotic activity, diagnosis of LMS was rendered. Immunohistochemistry showed diffuse positivity for smooth muscle actin [Table/Fig-4]. Other differential diagnosis which was considered was, a renal vein leiomyoma but increased mitotic activity and diffuse nuclear atypia favoured LMS. Another diagnosis which can mimic LMS here is sarcomatoid RCC, but due to absence of any attachment to renal parenchyma this diagnosis was excluded. Thus, correlating the site, histomorphology and immunohistochemistry, a diagnosis of renal vein LMS was rendered. Postoperative condition of patient was stable and was discharged from the hospital. Patient was doing well in her last follow up which was one month after the surgery.
H&E stained slides showed increased mitotic activity (both arrows and arrow head) (40X).
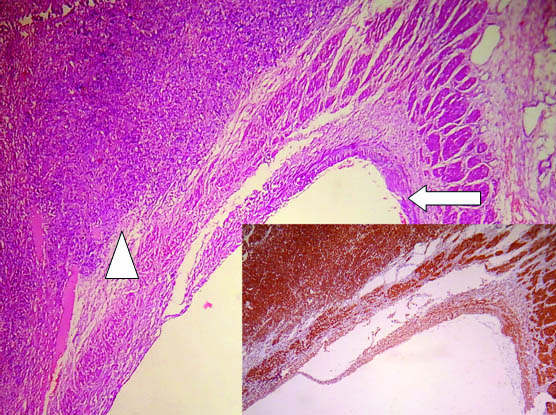
Low power view showing veinous wall at one end (arrow) and tumour arising from wall of renal vein at other end (arrow head). (H&E; 10X), inset shows diffuse positivity for SMA (10X).
Discussion
LMS account for <5% of soft tissue sarcomas [1]. LMS arising from venous channel are even rarer and more than half of cases involve inferior vena cava followed by long saphenous veins, femoral vein and internal jugular vein etc., in descending order of frequency [2,3]. LMS arising from renal vein is still rarer and according to literature till date less than 35 cases have been reported so far [4,5]. Renal vein LMS are more frequently seen in women and left kidney is more commonly involved. Various theories have been suggested regarding this clinical presentation. Female preponderance is supported by the theory that estrogenic stimulation leads to growth and proliferation of smooth muscle tumours. Involvement of left renal vein more than right is suggested by the longer length of left renal vein [6,7]. Symptoms are non specific with only few cases presenting with abdominal mass [8]. Some cases may be incidentally discovered.
Renal vein LMS is very difficult to diagnose preoperatively as radiologically it mimics RCC in view of close approximation of renal vein and kidney [9,10]. Our case also was originally diagnosed as operable RCC on radiology and was operated for the same.
On microscopy, the diagnosis was confirmed as LMS. Other differential diagnosis which were considered on this morphology was leiomyoma in view of absence of necrosis even after extensive sampling. However, increased mitotic activity and diffuse nuclear atypia favoured a diagnosis of LMS. IHC also confirmed the origin as smooth muscle with diffuse and strong SMA positivity. LMS may infiltrate perinephric fat and local recurrence is reported in 40% of cases. Metastasis may be seen in lungs or liver [11]. Our case did not show any infiltration of perinephric fat nor the distant metastasis. The preferred treatment for these patients is en block surgical resection including nephrectomy [6,12]. Chemotherapy may be offered to cases with metastasis disease. According to literature the prognosis is bad but overall survival is better than LMS of inferior vena cava [13].
Conclusion
Renal vein LMS is very difficult to diagnose preoperatively on radiology and should be considered as a differential diagnosis in any renal mass near to hilum of kidney. Very few cases have been reported in literature and thus limited availability of literature makes this case still important.
[1]. Shimoda HOK, Otani S, Hakozaki H, Yoshimura T, Okazaki HNS, Tomita S, Vascular leiomyosarcoma arising from the inferior vena cava diagnosed by intraluminal biopsyVirchows Arch 1998 8:97-100. [Google Scholar]
[2]. Fischer MG, Gelb AM, Nussbaum M, Haveson S, Ghali V, Primary smooth muscle tumours of venous originAnn Surg 1982 196(6):720-24. [Google Scholar]
[3]. Kevorkian J, Cento CP, Leiomyosarcoma of large arteries and veinsSurgery 1973 73:390-400. [Google Scholar]
[4]. Saltzman AF, Brown ET, Halat SK, Hedgepeth RC, An uncommonly encountered perirenal mass: Robotic resection of renal vein leiomyosarcomaCanadian Urological Association Journal 2015 9(3-4):E213-E216. [Google Scholar]
[5]. Devlin CM, Gill K, Thomas J, Biyani CS, Renal vein leiomyosarcoma and renal cell carcinoma presenting together: A case report and discussion on the follow upCanadian Urological Association Journal 2015 9(7-8):E517-E520. [Google Scholar]
[6]. Aguilar IC, Benavente VA, Pow-Sang MR, Morante CM, Meza L, Destefano V, Leiomyosarcoma of the renal vein: Case report and review of the literatureUrol Oncol 2005 23:22-26. [Google Scholar]
[7]. Deyrup AT, Montgomery E, Fisher C, Leiomyosarcoma of the Kidney- A clinicopathologic studyAm J Surg Pathol 2004 28:178-82. [Google Scholar]
[8]. Maturen KE, Vikram R, Wu AJ, Francis IR, Renal vein leiomyosarcoma and clinical features of a renal cell carcinoma mimicAbdom Imaging 2013 38:379-87. [Google Scholar]
[9]. Minami H, Ueki O, Tanaka T, Nishida H, Hashimoto T, Kawaguchi K, Case of leiomyosarcoma of the renal pelvisInt J Urol 2004 11:122-24. [Google Scholar]
[10]. Maeda T, Tateishi U, Fujimoto H, Kanai Y, Sugimura K, Arai Y, Leiomyosarcoma of the renal vein: Arterial encasement on contrast-enhanced dynamic computed tomographyInt J Urol 2006 13:611-12. [Google Scholar]
[11]. Cocuzza M, Arap S, Lucon AM, Saldanha LB, Renal leiomyosarcoma treated with partial nephrectomyClinics 2005 60:345-46. [Google Scholar]
[12]. Lemos GC, El Hayek OR, Apezzato M, Leiomyosarcoma of the renal veinInt Braz J Urol 2003 29:43-44. [Google Scholar]
[13]. Kato T, Yoneda S, Madono K, Tanigawa G, Fujita K, Yazawa K, Leiomyosarcoma of the renal vein: A case reportHinyokika Kiyo 2009 55(10):607-10. [Google Scholar]