Infections caused by CRE pose a global challenge in terms of management due to limited treatment options. Moreover, these strains pose an imminent danger of large scale dissemination in hospitals leading to a high burden of nosocomial infections. High fecal carriage of CRE is being witnessed in India and this is mostly related to poor infection control practices [3–5].
Tigecycline, the 9-t-butylglycylamido derivative of minocycline, is a broad spectrum bacteriostatic antibiotic and was the first glycylcycline antibiotic to be approved by the US Food and Drug Administration (FDA) in 2009 [6]. It is active against many multidrug resistant pathogens including Methicillin-Resistant Staphylococcus Aureus (MRSA), Vancomycin Resistant Enterococci (VRE), and CRE, although it lacks activity against Pseudomonas aeruginosa [7].
Low potentials for organ toxicity, fewer drug-drug interactions, convenient twice daily dosing and the lack of need to monitor renal function make the use of this antibiotic relatively uncomplicated. Parenteral use of tigecycline for the treatment of infections involving multidrug resistant pathogens has been increasing in India [8].
Although the resistance mechanisms seen in tetracycline like drug specific efflux pump acquisition and ribosomal protection are not seen with tigecycline, yet, clinical resistance has emerged recently [9,10]. Development of resistance to tigecycline during treatment of multidrug resistant bacteria like SHV-12-producing K. pneumoniae and KPC-3-producing E. coli has been noted and this may become more prevalent if it is used for infections such as UTI, septicaemia or abdominal abscess where subinhibitory concentrations of drug are achieved [11]. Resistance-Nodulation-cell Division (RND)-type transporters and other efflux pump systems associated with decreased tigecycline susceptibility have been found in clinical strains of E. coli and K. pneumonia [12]. Expression of efflux pump genes (acrA, acrB, tolC, oqxA and oqxB) and pump regulators (acrR, marA, soxS, rarA, rob and ramA) have been found associated with resistance to tigecycline in K.pneumoniae strains in various studies [13,14].
Judicious use of this antibiotic with strong clinical monitoring is necessary to preserve its effectiveness. There is an urgent need to collect and report data on MICs for tigecycline and this MIC data can be used both for surveillance purpose as well as for the management of individual cases. This study was conducted to know the trend of MICs for tigecycline in clinical isolates of E. coli and K. pneumoniae at our hospital.
Materials and Methods
A prospective observational study was carried out at the Department of Microbiology, Era’s Lucknow Medical College and Hospital (ELMCH); a 920 bedded Tertiary Care Hospital in Lucknow, Uttar Pradesh, India. The study was approved by the Institutional Ethics Committee at ELMCH. This was a one year study done from January 2015 till December 2015 and included various samples (including 830 sputum, 311 pus, 254 endotracheal aspirates, and 211 blood) from 1606 patients of all age groups admitted in various wards and ICUs of the ELMCH. Urine samples were excluded as tigecycline should not be used for UTIs due to poor urinary drug concentrations.
The clinical specimens were brought without delay to the laboratory and were processed according to standard microbiological techniques. Specimens of pus, sputum and endotracheal aspirates were directly cultured on blood agar, MacConkey and chocolate agar while blood cultures were done in BD BACTEC Plus Aerobic/F, Plus Anaerobic/F and Peds Plus/F media. E.coli and Klebsiellapneumonia were identified using conventional biochemical tests. Only one isolate per patient was included.
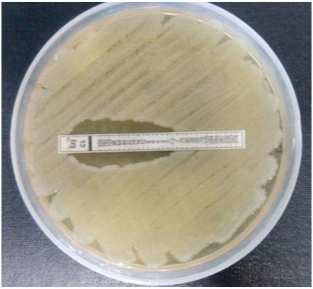
Antibiotic susceptibility testing: Susceptibility to imipenem, meropenem, ertapenem, amikacin, gentamycin, tobramycin, netilmycin, ciprofloxacin, levofloxacin, ceftriaxone, cefepime, piperacillin-tazobactam, ticarcillin-clavulanate, doxycycline and tigecycline was determined by the standard disk diffusion technique on Mueller-Hinton agar in accordance with the Clinical Laboratory Standards Institute (CLSI) 2015 guidelines using Hi media disks [15] [Table/Fig-1]. Isolates were designated as CRE when they were resistant to any of the three carbapenem antimicrobials tested [16]. Quality control was done using E.coli ATCC 25922. For testing colistin susceptibility, MICs were determined by E test using Ezy MIC TM CL strips from Hi Media. Breakpoints for colistin used were given by EUCAST 2016 with MIC >2 resistant and ≤2 considered to be susceptible [17].
Disk diffusion for E.coli showing resistance to tigecycline, imipenem, meropenem, amikacin, ciprofloxacin and piperacillin-tazobactam.
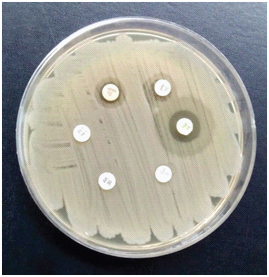
Susceptibility testing for tigecycline: In all isolates, tigecycline 15 μg (Hi-Media) disk was used to screen for tigecycline resistance. For disk diffusion testing, a zone of ≥18 was considered sensitive while ≤15 was considered as resistant [17].
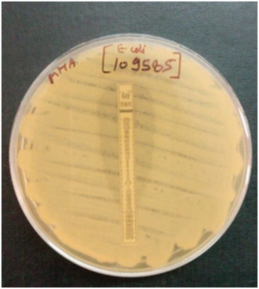
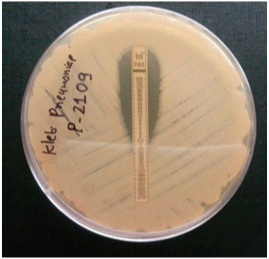
MICs of tigecycline were determined by E-test (Ezy MIC TM TG strips, Hi Media) according to manufacturer’s instructions. In view of the lack of tigecycline breakpoints for Enterobacteriaceae by CLSI, 2016 guidelines were used [17]. MIC breakpoints used were ≤1, 2 and >2 μg/ml for the susceptible, intermediate and resistant categories, respectively [Table/Fig-2,3 and 4].
E-test for tigecycline showing E.coli with MIC >2 μg/ml.
E-test for tigecycline showing K. pneumoniae with MIC =2 μg/ml.
E-test for colistin showing E.coli with MIC <2.
Results
During the one year study period, a total of 491 isolates of Enterobacteriaceae (275 E.coli and 216 K. pneumonia) were tested. These isolates were from various samples including 238 sputum, 116 pus, 103 endotracheal aspirates, and 34 blood cultures. The mean age of patients was 43 years and there were 312 male and 179 females. Samples from wards constituted 76% (374) while rest were from ICUs.
Sensitivity pattern of these two bacterial species shows a high level of resistance to most classes of antimicrobials [Table/Fig-5]. MICs of colistin were used to determine sensitivity and overall colistin was sensitive for 483 (98.4%) of isolates. Aminoglycosides and doxycycline also showed promising results in these multidrug resistant clinical isolates. Fluoroquinolones were least effective drugs especially for E.coli isolates with more than 81% of E.coli isolates being non-susceptible to both ciprofloxacin and levofloxacin. The sensitivity of tigecycline was 93.9% for K. pneumoniae and 97.4 for E.coli isolates by disk diffusion. The isolates recovered from patients admitted in ICU were more resistant to antibiotics.
Results of antibiotic susceptibility testing by disk diffusion showing isolates susceptible to various antibiotics. (n=491).
| ANTIBIOTIC(disk content in μg) | E.coliNo. of sensitive isolates (%) (n=275) | Klebsiella pneumoniaeNo. of sensitive isolates (%) (n=216) |
|---|
| Wards (224) | ICUs (51) | Wards (150) | ICUs (66) |
|---|
| Meropenem (10) | 191 (85.3) | 27 (52.9) | 124 (82.7) | 32 (48.5) |
| Imipenem (10) | 172 (76.8) | 21 (41.2) | 119 (79.3) | 31 (46.9) |
| Ertapenem (10) | 160 (71.4) | 16 (31.4) | 103 (68.7) | 26 (39.4) |
| Amikacin (30) | 140 (62.5) | 29 (56.8) | 83 (55.3) | 25 (37.8) |
| Gentamycin (10) | 121 (54) | 26 (51) | 62 (41.43) | 17 (25.8) |
| Tobramycin (10) | 116 (51.8) | 14 (27.4) | 70 (46.7) | 18 (27.3) |
| Netilmycin (30) | 111 (49.6) | 16 (31.4) | 71 (47.3) | 15 (22.7) |
| Ciprofloxacin (5) | 37 (16.5) | 6 (11.8) | 48 (32) | 13 (19.7) |
| Levofloxacin (5) | 41 (18.3) | 8 (15.6) | 60 (40) | 16 (24.2) |
| Ceftriaxone (30) | 35 (15.6) | 4 (7.8) | 28 (18.6) | 7 (10.6) |
| Cefepime (30) | 37 (16.5) | 6 (11.8) | 41 (27.3) | 16 (24.2) |
| Piperacillin/tazobactam(100/10) | 96 (42.8) | 11 (21.6) | 62 (41.3) | 18 (27.3) |
| Ticarcillin-clavulanate(75/10) | 73 (32.6) | 8 (15.7) | 59 (39.3) | 23 (34.8) |
| Doxycycline (30) | 77 (34.4) | 20 (39.2) | 68 (45.3) | 25 (37.9) |
| Tigecycline (15) | 219 (97.8) | 49 (96.1) | 143 (95.3) | 60 (90.9) |
| Colistin (Etest MIC) | 224 (100) | 50 (98) | 147 (98) | 62 (93.9) |
Out of 491 isolates tested, 186 (37.9%) were resistant to either of the three carbapenems tested and constituted the CRE isolates.
These included 99 E.coli and 87 K. pneumoniae. Distribution of CRE isolates in various samples show highest prevalence in pus 42.8%, followed by 35.3% in sputum, 32.3% in blood and 31% in endotracheal aspirate. A total of 40.3% of carbapenem resistant isolates were from patients admitted in ICUs and thus prevalence of carbapenem resistance was much higher (64.1%) when only ICU samples were considered.
Among the 186 CRE isolates, MIC testing for tigecycline was carried out in 144 isolates. We excluded 42 isolates due to financial constraints but these 42 isolates were all sensitive to tigecycline by disk diffusion with zones above 19 mm.
Tigecycline resistance was seen in 12 (8.3%) isolates (eight K. pneumoniae and four E. coli). Analysis of the MIC values shows that 12 isolates had MICs >2 and thus reported resistant to tigecycline, while eight isolates (five K. pneumoniae and three E. coli) had MIC value of two and reported intermediate [Table/Fig-6]. Thus, the overall prevalence of high MIC (≥2) of tigecycline was 20 (13.9%) in carbapenem resistant isolates of Enterobacteriaceae.
Distribution of tigecycline E-test MICs for carbapenem resistant isolates of E.coli and Klebsiella pneumonia.
| MIC of Tigecycline | Interpretation | No. of isolates |
|---|
| <=0.5 | Sensitive | 120 |
| 1 | Sensitive | 4 |
| 2 | Intermediate | 8 |
| 4 | Resistant | 10 |
| >=8 | Resistant | 2 |
(n=144)
Discussion
Carbapenemase producing Enterobacteriaceae were almost non existent in the 1990s. KPC is a class A β-lactamase which was first reported from a K. pneumoniae in North Carolina in USA in 2001 but has now spread worldwide [18]. The K. pneumoniae Carbapenemase (KPC) gene has been acquired by other Enterobacteriaceae including E.coli and Enterobacter species. New Delhi Metallo Beta Lactamase (NDM), a class B β-lactamase was first identified in 2008 in a K. pneumoniae isolate from a Swedish patient who had received medical care in India [19]. Since then it has spread more rapidly than KPC, in both healthcare and community settings in India. In a study from Mumbai, 22 of 24 consecutively collected CRE isolates contained the gene encoding NDM [20]. Other carbapenemases like VIM, OXA-48 have also been detected in hospitals all across India [21]. This is a global trend and like other developing countries the situation in India is worrisome.
In this study, carbapenem resistance was seen in 37.9% of all E.coli and K. pneumoniae isolated from clinical samples received from the hospitalized patients. The rate of carbapenem resistance was much higher (64.1%) in samples from patients admitted in various ICUs. This rate of carbapenem resistance is much higher than those reported in previous studies from India. In 2011 Dutta S et al., from Chandigarh, Punjab, India. in their study on neonatal septicaemia, reported 37.5 % and 9% meropenem resistance in isolates of E.coli and K. pneumoniae respectively [22].
A previous study in Mumbai, Maharashtra, India. reported a CRE prevalence rate of 12.26% with most of the isolates being detected in samples from the wards (42%) and the ICU (26%) [23]. Another study from New Delhi, India reported carbapenem resistance in 45% Klebsiella pneumoniae and 41% Escherichia coli isolates from ICU patients [24].
Kumar S and Bhadauria S reported 71.25% carbapenem resistance in 80 isolates of Enterobacteriaceae showing a rise in proportion from 65% to 85%. ICU patient’s positivity was found to be about 66% [25].
These CRE strains are resistant to all beta-lactams as well as to most other classes of antibiotics including quinolones, aminoglycosides. Going by the results of in vitro susceptibility tests, only two agents, tigecycline and polymyxins (polymyxin B and colistin) maintain good activity across most CRE. Combination regimens involving carbapenems in combination with colistin or high-dose tigecycline or aminoglycoside or even triple combinations may be used for therapeutic advantage over monotherapy [26]. For Enterobacteriaceae with carbapenem MICs higher than 8 mg/l, a combination of two or even three antibiotics among colistin, high-dose tigecycline, aminoglycoside and fosfomycin have been recommended [8].
Due to very limited treatment options, antimicrobials should be used judiciously and keeping in mind the results of in vitro sensitivity testing. Thus, a trend MICs must be known before considering any therapeutic regimen involving tigecycline [27].
Overall the sensitivity of tigecycline was good; being 93.9% for K. pneumoniae and 97.4 for E.coli isolates. Since this antibiotic is mostly used when possibility of carbapenem resistance is present, we analysed the MIC values in CRE isolates. The study shows an 8.3% of tigecycline resistance in CRE isolates and high MIC of 2 in another 8 (5.5%) of strains. The prevalence of high MIC (>=2) was thus seen in 13.9% of CRE tested including 13 K. pneumoniae and seven E.coli isolates.
In 2013, Sader HS et al., tested tigecycline activity against antimicrobial resistant surveillance subsets of clinical bacteria from a worldwide collection. They reported only four out of 213 (1.9%) meropenem-nonsusceptible Klebsiella species. to be tigecycline-nonsusceptible, all with tigecycline MIC of 4 μg/ml [28]. An Indian study by Chandran SP et al. reported tigecycline MIC90 of 2 in 42 NDM-1 producing Enterobacteriaceae resistant to carbapenems [29]. This warrants a careful approach when prescribing combined therapy especially for carbapenem resistant K. pneumoniae. The MIC testing is required, even if empirical therapy has been started so that possible treatment failures can be predicted and managed. Other antimicrobials including aminoglycosides, doxycycline or colistin have been used as combination therapy with/without carbapenems in managing CRE infections.
Tigecycline should be used cautiously in the hospitals, and emergence of efflux mediated resistance should be closely monitored in order to prolong the lifespan of this useful antibiotic. Local antibiograms and results of susceptibility testing must be taken in account while treating multidrug resistant bacteria like CRE.
Limitation
Our study should be interpreted in view of certain limitations. Due to financial constraints only 144 out of 186 CRE isolates could be subjected to E-test for determining MICs of tigecycline. There was also felt the need to confirm the non-susceptible tigecycline E-test results using a Broth Micro Dilution (BMD) method, but was not possible due to limitations of time and financial support.
Conclusion
Enterobacteriaceae isolates in our hospital are highly resistant to most common antimicrobials including carbapenems. Tigecycline MICs of ≥2 μg/ml in 20 (13.9%) of CRE isolates underscore the importance of in vitro sensitivity testing data in managing these infections. Tigecycline must not be used as monotherapy and for better optimization of individual therapy; combined testing may be carried out.
(n=144)