Meningitis is defined as the inflammation of the leptomeninges (the arachnoid mater and pia mater) [1]. The most common cause of inflammation is usually an infection of the Cerebrospinal Fluid (CSF) within the subarachnoid space. The most common cause of infection of the meninges includes viruses (aseptic meningitis), bacteria (pyogenic meningitis) and microorganisms like Mycobacterium tuberculosis (tuberculous meningitis) [1]. Certain factors such as age, immunosuppression, and neurosurgical procedures increase the chances of infection from specific pathogens.
Classical method for diagnosis of TBM includes demonstration of tuberculous bacilli in Ehrlich-Ziehl-Neelsen (EZN) stain of CSF and/or isolation of bacteria in CSF culture [3]. But these tests lack sensitivity and cultures takes more than six to eight weeks to show any evidence of growth and is often negative. Other tests based on nucleic acid analysis are available but cannot be used routinely, because of the high cost and specialized infrastructure involved. Therefore, for differentiation of TBM from non-TBM, a reliable and cost- effective test should be available. The determination of CSF-ADA activity may be a reliable and valuable adjunct in differentiating TBM from non-TBM in conjunction with other CSF parameters like mononuclear pleocytosis, high protein and low glucose.
Adenosine Deaminase (ADA) is an enzyme involved in purine metabolism. It catalyzes the deamination of adenosine and deoxyadenosine, forming inosine and deoxyinosine respectively, with release of ammonia [4]. Lymphocytic proliferation and differentiation is a major function of ADA. As a marker of cellular immunity, activity is found to be elevated in diseases producing a cell-mediated immune response, especially tuberculosis. The reliability of ADA activity in the CSF for early differential diagnosis of patients with TBM from those with non-TBM has already been established in prior studies [5]. CSF-ADA 10 U/l is the standard cut off value that is used for differentiation between TBM and non-TBM. In this study, we attempt to determine and validate a lower cut off value for CSF-ADA for diagnosing TBM in an Indian setting.
Materials and Methods
This was a prospective study involving 85 cases of meningitis whose CSF were analysed and ADA estimated using an enzymatic deamination assay kit. The study was approved by scientific and Ethical Committee of Amrita Institute of Medical Sciences, a tertiary care centre in Kochi, Kerala, India. The study was conducted from October 1st 2013 to April 30th 2015.
Inclusion Criteria
Subjects included in this study were all cases of suspected meningitis admitted to this hospital and were in the age group of 18-70 years. A suspected case of meningitis was defined as a symptom-complex of fever with headache and any of the associated symptoms like vomiting, neck stiffness, irritability, seizures, cranial nerve palsies, lethargy, altered consciousness or presence of papilloedema.
Exclusion Criteria
All cases of meningitis below 18 years and above 70 years of age, cases of partially treated meningitis and cases with a combination of TBM and non-TBM were excluded.
Diagnostic Criteria
TBM was defined as a case of suspected meningitis with any one positive confirmatory laboratory test or with a clinical score ≤ +4 [6,7] and any two supporting diagnostic criteria. All other cases were classified as non-TBM [Table/Fig-1].
Clinical Criteria for TBM [6,7].
| Variable | Score |
|---|
| Age (in years) | ≥ 36 | +2 |
| < 36 | 0 |
|---|
| Blood WCC (1000/ml) | ≥ 15000 | +4 |
| < 15000 | 0 |
| History of Illness | ≥ 6 days | -5 |
| < 6 days | 0 |
| CSF total WCC (1000/ml) | ≥ 750 | +3 |
| < 750 | 0 |
| CSF Neutrophils | ≥ 90% | +4 |
| < 90% | 0 |
Total score ≤ +4 is TBM, > 4 is non-TBM.
Confirmatory Laboratory Test for TBM
CSF Acid Fast Bacilli (AFB) smears and culture positivity for Mycobacterium tuberculosis confirm the diagnosis of TBM.
Supportive Diagnostic Criteria
CECT/MRI Brain showing exudates in basal cisterns/sylvian fissures, gyral enhancement, hydrocephalus, infarcts or tuberculoma, good response to Anti Tubercular Therapy (ATT) which is defined as complete resolution of the constitutional signs one month after treatment initiation, positive TB PCR (polymerase chain reaction) in CSF sample, CSF findings of high or normal protein with low glucose (<50% of corresponding blood sugar) and evidence of pulmonary TB (Sputum AFB positive or findings of chest radiograph) are considered to be supportive in the diagnosis of TBM.
Sample Collection and ADA Estimation:
CSF samples were obtained by lumbar puncture. About 3 ml of CSF was collected under sterile precautions. This was utilized for total and differential cell count, biochemical analysis, smears for Gram stain, Potassium Hydroxide (KOH) and AFB, as well as AFB culture and CSF TB PCR. The rest of the sample was utilized for ADA estimation. CSF-ADA activity was estimated in all 85 cases using adenosine deaminase assay kit provided by Diazyme laboratories and comparison of CSF-ADA in various types of meningitis was done.
Statistical Analysis
Results were expressed as mean±standard deviation for numerical variables. The comparison of mean value of CSF-ADA activity of the two types of meningitis was done using two sample t-test. Cut off value to differentiate between TBM and non-TBM meningitis was determined using ROC curve analysis. The sensitivity and specificity of the cut off value in diagnosing TBM was tested using McNemars Test. A p-value of <0.05 was considered as significant.
Results
A total of 85 cases of suspected meningitis satisfying diagnostic criteria were analysed in this study. Among the 85 cases of meningitis, 50 were males and 35 were females, with a male to female ratio of 1.4:1. The mean age of the study population was 45.06±14.58 years. Among the 85, 14(16.5%) were in the age group of 18-30 years, 37 (43.5%) were in the age group of 31-50 years and 34 (40%) were in the age group of >50 years.
Fever was the presenting symptom in 97.6%, headache in 85.9%, while vomiting and neck stiffness were seen in 84.7%. Cranial nerve palsies in 9.4% and seizure in 5.9% were observed.
In the study group, 34 were diagnosed as TBM and the remaining 51 were categorized as non-TBM. The mean ADA activity in CSF of TBM group was calculated as 10.97±4.43 and that of CSF in non-TBM group was calculated as 5.09±1.53. The mean CSF-ADA activity in TBM group was significantly higher than in non-TBM group, with a statistically significant p-value of <0.001 [Table/Fig-2].
Mean CSF-ADA levels in TBM and non-TBM.
| Diagnosis | N | Mean | SD | p-value |
|---|
| TBM | 34 | 10.97 | 4.429 | <0.001 |
| Non-TBM | 51 | 5.09 | 1.536 |
Two Sample t-test.
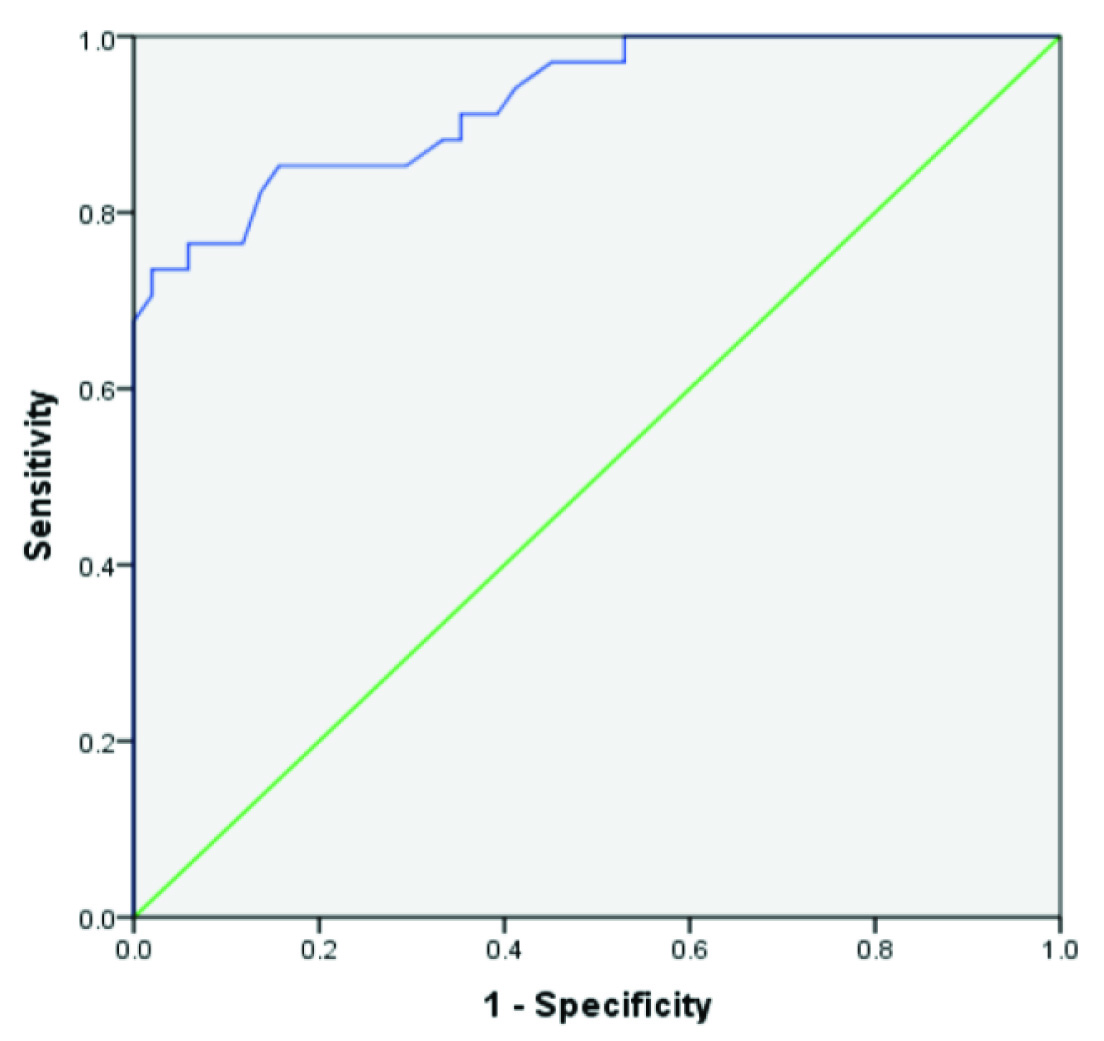
A cut off value of 6.65 was calculated using ROC curve for the diagnosis of TBM [Table/Fig-3]. Twenty nine cases of TBM had ADA above the cut off value (≥6.65) and five cases of TBM had ADA below the cut off value (<6.65). Eight cases of non-TBM had ADA above the cut off value and 43 cases had ADA below the cut off value [Table/Fig-4]. Taking 6.65 as the cut off value gave a sensitivity of 85.3% and a specificity of 84.3% for differentiating TBM from non-TBM. The positive predictive value was 78.3% and negative predictive value was 89.5% using the above cut off. The positive likelihood ratio was 5.44 and negative likelihood ratio was 0.17, when this lower cut off value was applied [Table/Fig-5].
Stratification based on ADA cut off value.
| CSF-ADA | TBM (n) | Non-TBM (n) | p-value |
|---|
| ≥ 6.65 | 29 | 8 | 0.581 |
| < 6.65 | 5 | 43 |
McNemars Test
| Measure | Value |
|---|
| Sensitivity | 85.3% |
| Specificity | 84.3% |
| Positive predictive value | 78.3% |
| Negative predictive value | 89.5% |
| Positive likelihood ratio | 5.44 |
| Negative likelihood ratio | 0.17 |
Discussion
TBM is still a major global health problem especially in the developing countries. It has high morbidity and mortality unless an early diagnosis is made and appropriate treatment is initiated. The use of CSF-ADA for differentiating TBM from non-TBM had been proven in various studies in the past. In this study, we have attempted to determine and validate a lower cut off value of CSF-ADA for diagnosing TBM in an Indian setting.
The mean ADA activity in CSF of TBM group was calculated as 10.97±4.43 and that in CSF of non-TBM group was calculated as 5.09±1.53. The mean CSF-ADA activity in TBM group was significantly higher than in non-TBM group, and it is statistically significant with a p-value of <0.001. In a similar study done by Kashyap RS et al., the mean ADA activity in TBM patients (14.31±3.87) was significantly higher than the non-TBM group (9.25±2.14 p< 0.0001 [5]. In another study by Aggarwal AK et al., the mean CSF-ADA activity in TBM was 13.58±4.54, in pyogenic meningitis was 7.68±2.00 and viral meningitis was 6.72±2.71 with a p-value of <0.001 [8]. In yet another similar study by Chander A et al., ADA levels (mean±SD) in the TBM and non-TBM groups were 16.46±6.24 U/l and 5.13±2.96 U/l, respectively [9].
In the present study, a cut off value of 6.65 was calculated using ROC curve for the diagnosis of TBM infection which gave a sensitivity of 85.3% and a specificity of 84.3% for differentiating TBM from non-TBM. The positive predictive value was 78.3% and negative predictive value of 89.5 % using the above cut off. The positive likelihood ratio was 5.44 and negative likelihood ratio of 0.17 when this lower cut off value was applied. In a study by Kashyap RS et al., with 117 cases of TBM and 60 cases of non-TBM, the sensitivity of the ADA test to differentiate between TBM and non-infectious meningitis was 82% and the specificity was 83% when the cut off value of 11.39 U/L/min was used [5]. In another study by Gautam N et al., with 20 TBM and 25 non-TBM cases, they determined a cut off value of 6.97 IU/l for diagnosis of TBM which gave a sensitivity of 85% and a specificity of 88% [10]. The sensitivity and specificity of CSF-ADA in diagnosing TBM from this study was comparable to the present study. In yet another similar study by Chander A et al., with a cut off value of 10 IU/l, they have looked at positive likelihood ratio (9.03%) and negative likelihood ratio (0.19%) in addition to the sensitivity (82.14%) and specificity (90.91%) [9]. The positive and negative likelihood ratio which was calculated in the present study is comparable despite a lower cut off value and hence, adds significant weightage to the pretest probability of either of the diseases.
Limitation
In our attempt to determine and validate a lower cut off value of CSF-ADA we have only observed a positive likelihood ratio and negative likelihood ratio of 5.44 and 0.17 respectively. This translates to only a moderate increase or decrease in likelihood of TBM. This might be because of the smaller sample size in this study. The results might still be widely applicable in conjunction with the other standard tests as they form a cost-effective option which will help in early initiation of treatment.
Conclusion
This study has demonstrated that CSF-ADA can be used as an important diagnostic tool in early diagnosis of TBM. A lower cut off value of 6.65 for CSF-ADA in TBM gave a good sensitivity and specificity in differentiating it from non-TBM. The positive likelihood Ratio of 5.44 shows a moderate increase of likelihood of TBM and a negative likelihood ratio of 0.17 also shows a moderate decrease of likelihood of TBM using this cut off value of CSF-ADA. Hence, a lower cut off value of CSF-ADA at 6.65, in conjunction with other CSF parameters like mononuclear pleocytosis, high protein and low glucose, is a rapid, easily available and cost-effective diagnostic test that helps in differentiating TBM from non-TBM.
Two Sample t-test.
McNemars Test