Acute hepatitis can be due to a number of causes. Acute inflammation of the liver usually recovers in a few months [1,2]. Acute hepatitis sometimes progress to chronic hepatitis, and it rarely leads to acute liver failure. The most frequent cause of acute hepatitis is infection (especially, acute viral hepatitis). It may also occur as a result of use of some drugs and/or herbals, overdose of drugs, exposure to toxic chemicals (alcohol) [3,4]. Other causes of acute hepatitis are autoimmune hepatitis and mushroom poisoning. Symptoms vary from mild to severe with fatigue, pain, itching, fever, and jaundice. Liver function tests (LFT) are used for diagnosis. ALT should be 10-50 times higher than normal limits (for ALT >2,000 IU, drug history should be considered, acetaminophen level if appropriate). Elevated AST, GGT and total bilirubin levels could be observed in patients with acute hepatitis. Healing process usually occurs in patients with acute hepatitis within a few months. Acute hepatitis may sometimes deteriorate and a liver transplant may be needed for improvement [5,6].
FABPs are a family of 15-kDa proteins. There are nine different FABPs that have been identified and named according to the tissues [7]. L-FABP is expressed mainly in the liver, but small quantities are also found in the kidney and small intestine [7,8]. Till date, a large number of studies on different types of FABPs have shown that these proteins are associated with tissue damage, including myocardial injury, and damage to other organs such as lungs, kidneys, intestine and liver [9-17].
L-FABP is a small molecule that is expressed in the liver. Till date, some studies including chronic hepatitis C, non-alcoholic steatohepatitis and non-alcoholic fatty liver disease have shown that, serum L-FABP could be a new diagnostic marker to detect liver injury [18-20].
Hence, we aimed to investigate the relationship between serum/urine L-FABP levels and liver damage in patients with acute hepatitis and determination of diagnostic value in prediction of liver damage.
Materials and Methods
This was a prospective study. This study was administrated at the gastroenterology clinic of an Necmettin Erbakan University, Meram school of Medicine Hospital. The study protocol was approved by the local Ethical Committee. All patients or first degree relatives and control subjects signed informed consent forms. Consecutive patients with acute hepatitis, hepatic encephalopathy and stable cirrhosis who were admitted to gastroenterology polyclinic or clinic were enrolled for evaluation of suitability for the study between March, 2012 and March, 2013. The exclusion criteria were as follows: malignancy, hepatocellular carcinoma, active cerebrovascular event, shock, trauma, active infection, inflammatory diseases, renal diseases, use of nephrotoxic drug, intoxication, metabolic disorders, brain disorders, psychiatric diseases, use of sedatives or psychiatric drugs.
Participants
Four groups of patients were included in our study. Group 1 consisted of patients with acute hepatitis. These patients had no previous liver disease. Their transaminases were 10-50 times higher than the normal limits. We enrolled patients who had ALT above 300 IU. Group 2 consisted of patients with overt hepatic encephalopathy. These patients also had a clinical and/or histopathologic diagnosis of cirrhosis. These patients had overt hepatic encephalopathy throughout their hospitalization. We evaluated grades of hepatic encephalopathy based on West Haven criteria for semiquantitative grading of mental state. These grades are as follows: Grade 1- trivial lack of awareness, euphoria or anxiety, shortened attention span, impaired performance of addition; Grade 2- lethargy or apathy, minimal disorientation for time or place, subtle personality change, inappropriate behaviour, impaired performance of subtraction; Grade 3-somnolence to semi-stupor (but responsive to verbal stimuli), confusion, gross disorientation; Grade 4-coma (unresponsive to verbal or noxious stimuli) [21]. Group 3 consisted of patients with stable cirrhosis. These patients had diagnosis of cirrhosis but had no serious complication of cirrhosis such as hepatorenal syndrome, bleeding of varix, hepatic encephalopathy, or hepatopulmonary syndrome. Group 4 consisted of control subjects. These were the subjects who came for check-up at the gastroenterology polyclinic. They did not have any disease.
An ultrasonographic evaluation was performed for severity of ascites as mild, moderate or severe. Grades of ascites were as follows: mild ascites (Grade 1) – only detectable by ultrasound examination; moderate ascites (Grade 2) – that is manifested as moderate symmetrical distension of the abdomen; severe ascites (Grade 3) – with marked abdominal distension [22].
Clinicodemographic Data and Collection of Blood Samples
Blood and urine samples were obtained from patients and control subjects in the all groups for analysis. Each patient who was suitable for inclusion in the study underwent a physical examination, and detailed medical history was recorded. Serum and urine L-FABP levels were analysed from collected samples.
Serum and urine samples were centrifuged at 4°C and 1,000 g for 15 minutes. These samples were stored at -80°C until analysis. Blood and urine samples were analysed by biochemists using Human L-FABP ELISA kit (L-FABP, E91566Hu, USCN Life Science Houston, TX, USA). Briefly, microtitre plates were coated with a monoclonal antibody against human L-FABP and 100 μL serum and urine samples were added and incubated for one hr at 37°C. After washing, each one was incubated with 100 μl biotinylated monoclonal antibody for one hour at 37°C. The solution was aspirated and washed three times, followed by addition of 100 μl avidin-conjugated HRP and incubation for one hour at 37°C. After washing five times, 100 μl tetramethylbenzidine substrate was added for colour development in the dark room, which was read after 10-30 min at 450 nm using an ELISA reader (Benchmark Plus, BioRad, Hercules, CA, USA).
Statistical Analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) for Windows, version 11.5 (SPSS Inc., Chicago, IL, United States). Whether the continuous variables were distributed normally or not was determined by Kolmogorov Smirnov test. Levene test was used for the evaluation of homogeneity of variances. Data were expressed as median (IQR).
Whether the differences in medians among groups were statistically significant or not was evaluated by Kruskal Wallis test. When the p-value from Kruskal Wallis test was statistically significant, Conover’s multiple comparison test was used to find which group differed from the others. Categorical data were analyzed by Pearson’s chi-square test. Degrees of association between continuous variables were evaluated by Spearman’s rank correlation analyses.
The optimal cut-off points of serum and urinary L-FABP to discriminate controls from other case groups were evaluated by ROC analyses as giving the maximum sum of sensitivity and specificity for the significant test. Diagnostic performance (i.e. sensitivity, specificity, positive and negative predictive values) for each clinical measurement were also calculated.
A p-value less than 0.05 was considered statistically significant. For all possible multiple comparisons the Bonferroni Correction was applied for controlling type I error.
Results
A total of 85 patients with 17 of acute hepatitis (five of acute hepatitis B, one of acute hepatitis A, two of acute hepatitis C, five of autoimmune hepatitis and four of toxic hepatitis), 19 of hepatic encephalopathy, 29 of liver cirrhosis, and 20 control subjects were included in the study.
There was no statistically significant difference between the groups in terms of gender (p=0.307). There was no statistically significant difference between acute hepatitis and control groups in terms of age (p>0.05). Demographic and clinical features are shown at [Table/Fig-1].
Demographic and clinical features.
| Variables | Acute Hepatitis (n=17) | Hepatic Encephalopathy (n=19) | Stable Cirrhosis (n=29) | Control (n=20) | p-value |
|---|
| Age | 48.8±18.2a,b | 65.3±13.9a,c | 63±12.8b,d | 42.7±16.9c,d | <0.001† |
| Gender | 0.307‡ |
| Female | 8 (47.1%) | 7 (36.8%) | 10 (34.5%) | 12 (60%) |
| Male | 9 (52.9%) | 12 (63.2%) | 19 (65.5%) | 8 (40%) |
| AST | 453±395.5a,b,e | 67 ±60a,c | 47±28.5b,d | 15±5.5c,d,e | <0.001† |
| ALT | 616±437a,b,e | 44±36a,c | 29±19b,d | 14.5±9c,d,e | <0.001† |
| CRE | 0.69±0.26a | 1.21±1.19a,c,f | 0.80±0.38d,f | 0.60±0.24c | <0.001† |
| GGT | 140±131a,b,e | 50±66a,c | 38±32.5b,d | 21±6.25c,d,e | <0.001† |
| Serum L-FABP | 9110±3352.5e | 9410±1355c | 9715±2462.5d | 3672±982.5c,d,e | <0.001† |
| Urine L-FABP | 8730±1155a,b,e | 9896±1108a,c | 9350±854b,d | 3391.5±312.5c,d,e | <0.001† |
† Kruskal Wallis test, ‡ Pearson’s Chi-square test
a: There was statistically significant difference between acute hepatitis and hepatic encephalopathy (p<0.01)
b: There was statistically significant difference between acute hepatitis and stable cirrhosis (p<0.01)
c: There was statistically significant difference between hepatic encephalopathy and control subjects (p<0.05)
d: There was statistically significant difference between stable cirrhosis and control subjects (p<0.001)
e: There was statistically significant difference between acute hepatitis and control subjects (p<0.001)
f: There was statistically significant difference between HE and SC (p<0.001).
Serum L-FABP levels were 9110±3352.5, 9410±1355, 9715±2462 and 3672±982.5 ng/l in patients with acute hepatitis, hepatic encephalopathy, cirrhosis and control subjects, respectively.
Urine L-FABP levels were 8730±1155, 9896±1108, 9350±854.5 and 3391.5±312.5 ng/l in patients with acute hepatitis, hepatic encephalopathy, cirrhosis and control subjects, respectively.
ALT levels in patients with acute hepatitis was significantly higher than hepatic encephalopathy and stable cirrhosis, and control subjects, respectively (p<0.001, p<0.001 and p<0.001).
AST levels in patients with acute hepatitis was significantly higher than hepatic encephalopathy and stable cirrhosis, and control subjects, respectively (p=0.002, p<0.001 and p<0.001).
Serum Cre levels in patients with hepatic encephalopathy was significantly higher than acute hepatitis and stable cirrhosis, and control subjects, respectively (p<0.001, p<0.001 and p<0.001). Therefore, Cre levels in patients with stable cirrhosis was significantly higher than control subjects (p=0.015). There was no statistically significant difference between acute hepatitis and control groups in terms of Cre levels (p>0.05).
GGT levels in patients with acute hepatitis was significantly higher than hepatic encephalopathy and stable cirrhosis, and control subjects, respectively (p<0.01, p<0.01 and p<0.001).
Serum L-FABP levels in patients with acute hepatitis, hepatic encephalopathy and stable cirrhosis were significantly higher than control subjects, respectively (p<0.001, p<0.001 and p<0.001) [Table/Fig-2].
Serum and urine L-FABP levels in groups.
Serum and urine L-FABP levels in patients with acute hepatitis, hepatic encephalopathy and stable cirrhosis were significantly higher than control subjects (*p<0.05). Urine L-FABP levels in patients with hepatic encephalopathy and stable cirrhosis were significantly higher than patients with acute hepatitis, respectively (* p<0.001 and ∆ p=0.006).
*AH: Acute Hepatitis, HE: Hepatic Encephalopathy, SC: Stable Cirrhosis, L-FABP: Liver Fatty Acid Binding Protein
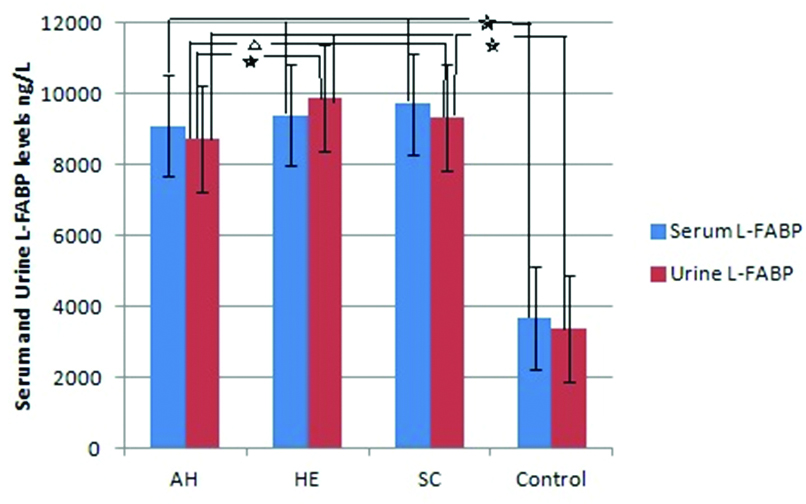
Urine L-FABP levels in patients with acute hepatitis, hepatic encephalopathy and stable cirrhosis were significantly higher than control subjects, respectively (p<0.001, p<0.001 and p<0.001). At the same time, urine L-FABP levels in patients with hepatic encephalopathy and stable cirrhosis were significantly higher than patients with acute hepatitis, respectively (p<0.001 and p=0.006) [Table/Fig-2].
There were statistically significant strong positive correlation between serum L-FABP levels and AST, ALT, Cre and GGT (p<0.001). There were statistically significant positive correlation between urine L-FABP levels and AST, ALT, Cre and GGT (p<0.05). Correlation of several laboratory parameters with serum and urine L-FABP levels are shown in [Table/Fig-3].
Correlation analysis between L-FABP and laboratory parameters.
| Serum L-FABP | Urine L-FABP |
|---|
| r | p† | r | p† |
|---|
| AST | 0.467 | <0.001 | 0.400 | <0.001 |
| ALT | 0.348 | <0.001 | 0.326 | 0.002 |
| CRE | 0.378 | <0.001 | 0.256 | 0.018 |
| GGT | 0.471 | <0.001 | 0.321 | 0.003 |
† Spearman’s Rank Correlation
AST: Aspartate transaminase, ALT: Alanine aminotransferase, CRE: Creatinine, GGT: Gamma glutamyl transferase
ROC curve analysis showed that the plot of the serum L-FABP and urine L-FABP, could be the diagnostic marker of liver damage (AUC=0.985 and AUC=1.000, respectively) [Table/Fig-4].
ROC curve plots of the serum L-FABP and urine L-FABP in diagnostic marker of liver damage.
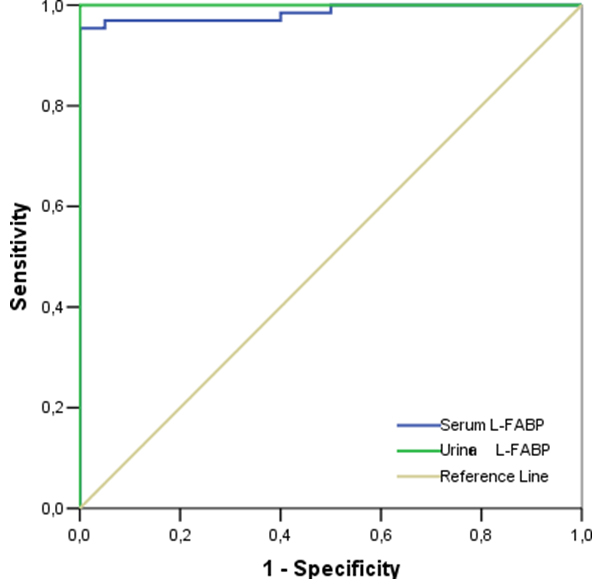
While cut-off value of serum L-FABP was 5183 ng/l for all of the patients, the sensitivity and specificity were 95.4% and 100%, respectively. Positive and negative predictive values for serum L-FABP were 100% and 87%, respectively. While cut-off value of urine L-FABP was 4367 ng/l for all of the patients, the sensitivity and specificity were 100% and 100%, respectively. Positive and negative predictive values for urine L-FABP were 100% and 100%, respectively. A comparison between groups and control are shown in [Table/Fig-5,6].
A comparison between each group and control in prediction of diagnostic marker of serum L-FABP levels.
| Cut- off | AUC (95%CI) | Sens. % | Spec. % | PPV% | NPV% | p-value |
|---|
| All Patients (serum L-FABP) | >5183 | 0.985 (0.965-1.000*) | 95.4% | 100% | 100% | 87% | <0.001 |
| AH (serum L-FABP) | >4818 | 0.944 (0.868-1.000*) | 88.2% | 95% | 93.8% | 90.5% | <0.001 |
| HE (serum L-FABP) | >5875.5 | 1.000 (1.000-1.000) | 100% | 100% | 100% | 100% | <0.001 |
| SC (serum L-FABP) | >5415.5 | 1.000 (1.000-1.000) | 100% | 100% | 100% | 100% | <0.001 |
*95% CI upper limit>1.000
AUC, area under the curve; CI, confidence interval; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, Negative predictive value
AH: Acute Hepatitis, HE: Hepatic Encephalopathy, SC: Stable Cirrhosis
A comparison between each group and control in prediction of diagnostic marker of urine L-FABP levels.
| Cut-off | AUC (95%CI) | Sens. % | Spec. % | PPV% | NPV% | p-value |
|---|
| All patients (urine L-FABP) | >4367 | 1.000 (1.000-1.000) | 100% | 100% | 100% | 100% | <0.001 |
| AH (urine L-FABP) | >5294.5 | 1.000 (1.000-1.000) | 100% | 100% | 100% | 100% | <0.001 |
| HE (urine L-FABP) | >5359.5 | 1.000 (1.000-1.000) | 100% | 100% | 100% | 100% | <0.001 |
| SC (urine L-FABP) | >4367 | 1.000 (1.000-1.000) | 100% | 100% | 100% | 100% | <0.001 |
AH: Acute Hepatitis, HE: Hepatic Encephalopathy, SC: Stable Cirrhosis
AUC: area under the curve; CI: confidence interval; Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: Negative predictive value
Discussion
This is the first prospective study that investigates the cut-off level of serum and urine L-FABP for diagnosing liver damage in patients with acute hepatitis, hepatic encephalopathy and stable cirrhosis. Our data suggest that serum and urine L-FABP is able to diagnose liver damage. This study showed good correlation between serum and/or urine L-FABP levels and AST, ALT, Cre, and GGT.
A previous study showed that L-FABP is a diagnostic marker for the detection of early hepatocellular injury in liver transplant patients and indicated that L-FABP is a small molecule that was elevated early in association with acute liver injury. Therefore, L-FABP appears to be superior to other liver enzymes including ALT, AST and glutathione S-transferase (GST) [23]. Our study showed that L-FABP has some advantages over other liver enzymes, because L-FABP is elevated in all types of liver damage, whereas the others are not.
A study showed a correlation between Knodell score and L-FABP, but there was no correlation between Knodell score with ALT or AST. Therefore, it was indicated that L-FABP could be superior to ALT and AST [18]. Another study suggested that elevation of serum L-FABP levels were associated with the degree of fibrosis and inflammation, indicating that serum L-FABP could be a non-invasive marker in determining the severity of fibrosis and inflammation in patients with non-alcoholic steatohepatitis [19]. One study demonstrated that elevated serum L-FABP levels were related to ongoing liver damage in patients with non-alcoholic fatty liver disease [20]. Our study provided a comparison between patients with acute hepatitis, cirrhosis and controls based on serum and urine L-FABP levels as a diagnostic marker. According to our data, serum and urine L-FABP are useful in detecting liver damage.
In a previous study, L-FABP levels increased significantly during liver mobilization that is associated with hepatocellular damage and liver inflammation [17]. Some studies showed elevated L-FABP levels in patients with Pringle manoeuvre during liver surgery; whereas, aminotransferases did not change significantly [24,25]. One study showed that plasma levels of L-FABP correlated well with warm ischaemia and concomitant hepatocellular damage in liver transplantation from non-heart-beating donors. Therefore, they said that monitoring of post-transplant L-FABP plasma levels was a valuable new tool to quantify early the extent of parenchymal cell damage of non-heart-beating donor liver and to predict their viability and function [26].
Other mechanisms of liver damage involve the toxic effects of alcohol and drugs. A previous study showed that alcohol consumption caused a significant increase in serum I- and L-FABP levels, compared to water consumption [27]. Another study described the hepatotoxic effects of pyrazinamide and the role of the Peroxisome Proliferator-Activated Receptor Alpha (PPARα) and its target genes in the downstream pathway including L-FABP, Lpl, Cpt-1b, Acaa1, Apo-A1 and Me1 in this process. At the same time, they found that pyrazinamide induced the liver lipid metabolism disorder and PPARα expression was down-regulated which had a significant inverse correlation with liver injury degree [16].
Limitation
There are some limitations in our study. Firstly, the number of patients is relatively small. Larger studies should be considered to clarify diagnostic value of serum and urine L-FABP in liver damage. Secondly, there are significantly difference between hepatic encephalopathy, stable cirrhosis and control groups in terms of age but not in acute hepatitis and control groups.
Conclusion
Our study showed that serum and urine L-FABP levels were strongly correlated with liver damage. Therefore, these biomarkers could be a non-invasive diagnostic marker for liver damage. Our study suggested that when the liver is damaged L-FABP is always elevated, but other liver enzymes may found to be normal. Therefore, estimation of L-FABP should be a priority compared to other liver enzymes, and could be used for followup of the progress of liver damage.
Financial Support: This study was supported by Necmettin Erbakan University Scientific Research Projects Fund.
† Kruskal Wallis test, ‡ Pearson’s Chi-square test
a: There was statistically significant difference between acute hepatitis and hepatic encephalopathy (p<0.01)
b: There was statistically significant difference between acute hepatitis and stable cirrhosis (p<0.01)
c: There was statistically significant difference between hepatic encephalopathy and control subjects (p<0.05)
d: There was statistically significant difference between stable cirrhosis and control subjects (p<0.001)
e: There was statistically significant difference between acute hepatitis and control subjects (p<0.001)
f: There was statistically significant difference between HE and SC (p<0.001).
† Spearman’s Rank Correlation
AST: Aspartate transaminase, ALT: Alanine aminotransferase, CRE: Creatinine, GGT: Gamma glutamyl transferase
*95% CI upper limit>1.000
AUC, area under the curve; CI, confidence interval; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, Negative predictive value
AH: Acute Hepatitis, HE: Hepatic Encephalopathy, SC: Stable Cirrhosis
AH: Acute Hepatitis, HE: Hepatic Encephalopathy, SC: Stable Cirrhosis
AUC: area under the curve; CI: confidence interval; Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: Negative predictive value