RA is a systemic, chronic inflammatory disorder most commonly affecting the musculoskeletal system. It is characterized by symmetrical deforming polyarthritis that predominantly affects small joints such as the metacarpophalangeal, interphalangeal and metatarsophalangeal joints [1-3]. According to WHO estimates, the worldwide prevalence of RA varies between 0.3% and 1%, with a female predominance [4]. Studies in the Indian population have shown prevalence rates as high as 0.75%, giving a total of more than seven million patients of RA [5]. Globally, RA is the 42nd highest contributor to disability, ranking just below malaria [6]. The impact of the disease is reflected in the fact that within 10 years of onset, 50% of patients are unable to hold a full time job [7].
Apart from the joints, RA has also been implicated in involvement of skin, eye, heart, nervous and gastrointestinal systems. Being an inflammatory condition, RA affects the cardiovascular system in the form of increased intimo-medial thickness, endothelial dysfunction and in general, a higher prevalence of atherosclerosis. These extra-articular manifestations have been found to be strong predictors of decreased survival in patients of RA [8]. Autonomic Nervous System (ANS) involvement in patients of RA has been reported variably by different authors. Discrepancies may be attributable to the use of different modes of autonomic testing and inconsistencies in the criterion of abnormality [9]. AN may present as sweating disturbances, gastrointestinal symptoms such as post-prandial fullness, diarrhoea or constipation, bladder dysfunction, deranged breathing control or erectile dysfunction [10]. These effects, though subtle, significantly affect the patient’s quality of life. Cardiovascular Autonomic Neuropathy (CAN) which is associated with deranged heart rate control and vascular dynamics has been linked to serious complications like myocardial infarction, arrhythmias and sudden cardiac death [11].
Various mechanisms have been proposed for the pathogenesis of AN in patients with RA. Maule S et al., suggested that autoantibodies directed against components of the ANS may play a role in the development of AN [9]. Altawil R et al., correlated heart rate variability with serum and Cerebrospinal Fluid (CSF) levels of Interleukin (IL)-1β and IL-6 and concluded that elevated intra thecal levels of the pro-inflammatory cytokine IL-1β may be responsible for the low vagal activity [12]. Vasculitis of the vasa nervorum (small arteries supplying blood to the peripheral nerves) and amyloidosis have also been linked to nerve dysfunction [13,14]. In future, these pathways could be targeted to prevent or halt the progression of AN.
With growing literature from around the world authenticating the involvement of the ANS in RA, we considered it prudent to investigate further the prevalence of AN in an Indian setting, data about which remains scarce. As AN may affect the quality of life and prognosis in RA patients, we tried to correlate it with the demographic (age and sex) and disease characteristics (severity, duration and serological status) of patients. If found, the correlation may help clinicians to suspect and thus, mitigate the harmful effects of ANS dysfunction in patients of RA.
The aims of the study were to find out the prevalence and severity of autonomic neuropathic dysfunction in adult patients of RA and to compare the results with a group of healthy volunteers and also to correlate the same with the patient’s age, gender, disease severity, disease duration and serological status.
Materials and Methods
Study Design
This cross-sectional study was conducted in a tertiary care hospital, New Delhi, India, during the period from June, 2015 to August, 2015. A sample of convenience with a minimum of 30 patients and an equal number of age and sex matched healthy volunteers were considered for this study.
Patient Group: Adult patients diagnosed with RA as defined by the American College of Rheumatology, 2010, criteria and receiving regular healthcare from the outpatient Rheumatology Clinic were included.
Healthy Group: Age and sex matched healthy adult volunteers from amongst the patient attendants and hospital staff were also recruited for comparison. An age difference of less than or equal to three years was considered as the criterion for age matching.
Exclusion Criteria: Patients of RA who were also suffering from conditions like hypertension, ischaemic heart disease, heart failure, cardiomyopathy, valvular heart disease, anaemia (haemoglobin<10 gm/dl), kidney disease, liver disease, thyroid dysfunction and other possible causes of neuropathy like diabetes mellitus were excluded. Pregnant patients and those currently on drugs that could affect the ANS like vasodilators, beta blockers, anti arrhythmics, sedatives and anti depressants were excluded from the study.
Ethics Considerations: Clearance was taken from the Institutional Ethics Committee (IEC) prior to commencement of the study. The subjects were provided with a subject information sheet along with a consent form, in both English and Hindi. Written informed consent was obtained from each participant before inclusion.
Methodology: Demographic information, baseline supine BP and HR of all participants was recorded in a standardized Case Report Form (CRF). An ECG to confirm normal sinus rhythm was performed using a standard electrocardiogram machine (BPL Cardiart 6108T). Disease duration (time since first diagnosis) and serological status were noted from the patient’s case records. Severity of RA was assessed using the Clinical Disease Activity Index (CDAI) score which takes into account the number of swollen and tender joints as well as the patient’s and the evaluator’s overall assessment. On the basis of the CDAI score, the patients were divided into four groups- remission, low disease activity, moderate disease activity and high disease activity.
Questionnaire: For the evaluation of autonomic neuropathy, the Survey of Autonomic Symptoms (SAS) scale devised and validated by Zilliox L et al., was used [15]. SAS covers the domains of orthostatic, sudomotor, vasomotor, gastrointestinal, urinary and sexual dysfunction in the form of 11 ‘yes’ or ‘no’ questions for females and 12 for males. The total number of symptoms was represented by the SAS-A score. Further, a positive response to each question was assigned a score from one to five. The sum of these responses, representing the total impact score was recorded as the SAS-B score.
Cardiovascular Autonomic Testing
Status of the cardiovascular reflexes was assessed by a battery of five non-invasive tests as described previously by Ewing DJ and Clarke BF [16]. Reporting for the tests is defined in [Table/Fig-1]. Grading of cardiac AN described in [Table/Fig-2], was done according to the description by Agarwal AK et al., [17]. To avoid ambiguity, borderline test results were not considered as abnormal for grading purposes.
Reporting of cardiovascular autonomic tests.
| Normal | Borderline | Abnormal |
|---|
| 1. HR response to Valsalva maneuver (Valsalva ratio) | >1.21 | 1.11-1.20 | <1.10 |
| 2. HR variability with deep breathing (maximum – minimum heart rate) | >15 beats/min | 11-14 beats/min | <10 beats/min |
| 3. HR response to standing (30:15 ratio) | >1.04 | 1.01-1.03 | <1.00 |
| 4. BP Response to standing (Fall in systolic BP) | <10 mmHg | 11-29 mmHg | >30 mmHg |
| 5. BP response to sustained handgrip (increase in diastolic BP) | >16 mmHg | 11-15 mmHg | <10 mmHg |
| Grade | Criteria | Severity of Autonomic Neuropathy |
|---|
| 0 | No abnormal test | Nil |
| I | One abnormal test | Mild |
| II | Two abnormal tests | Moderate |
| III | Three or more abnormal tests | Severe |
1. Heart Rate Response to Valsalva Manoeuvre: The subject, sitting comfortably, was asked to maintain an expiratory pressure of 40 mmHg for 15 seconds by blowing into a mouthpiece connected to a sphygmomanometer (Diamond Clock Model- BPDL 237). An ECG was taken during and after the manoeuvre. The Valsalva ratio was then calculated by dividing the maximum R-R interval after the manoeuvre by the minimum R-R interval during the maneuver.
2. Heart Rate Variability with Deep Breathing: The subject, in supine position, was asked to breathe deeply at the rate of six times per minute for one minute. An ECG was recorded continuously throughout the period and the onset of each inspiration and expiration was marked. The maximum and minimum R-R intervals were measured and converted into beats/minute. The results were then expressed as the mean of the difference between the maximum and minimum HR over the six cycles.
3. Heart Rate Response to Standing: An ECG machine was attached to the subject, while he/she was supine, and readings were taken after the patient switched to the vertical position without any aid. The result was quantitated using the 30:15 ratio which was obtained by dividing the R-R interval at the 30th beat after standing (time of maximum bradycardia) by the R-R interval at the 15th beat after standing (time of maximum tachycardia).
4. Blood Pressure Response to Standing: The subject was asked to lie down for 10 minutes, followed by standing for three minutes. The systolic blood pressure was measured first just before standing and then three minutes after standing. The difference between the two readings was taken as the postural fall in BP.
5. Blood Pressure Response to Sustained Handgrip: BP was recorded just before and during sustained handgrip performed using a hand-grip dynamometer (Qingfeng Hand Dynamometer). The subject was asked to maintain 30% of the isometric maximum for five minutes. The absolute difference between the diastolic blood pressure during and before the handgrip was calculated.
Statistical Analysis
Data ultimately recorded in the case report form was analyzed using Microsoft Excel and Statistical Package for the Social Sciences (SPSS) version 20.0. Prevalence of AN between RA patients and healthy volunteers was compared using the chi-square test, with the null hypothesis ‘there is no difference in prevalence of AN between patients of Rheumatoid Arthritis and healthy subjects’. Continuous data was taken as a mean with standard deviation and categorical data was taken as a percentage. Correlation analyses were performed using the Pearson Correlation Index. Statistical significance in all tests was taken as p<0.05.
Results
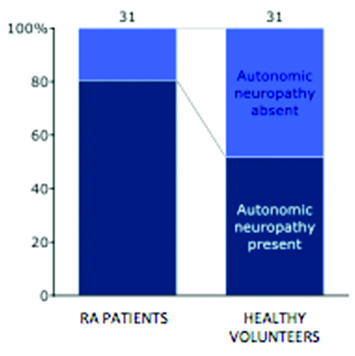
A total of 39 patients from the Rheumatology Clinic diagnosed with RA and fulfilling the inclusion criteria were initially recruited. Out of them, eight were subsequently excluded due to lack of complete data. Finally, there were a total of 31 patients with five males and 26 females. An equal number of age and sex matched healthy volunteers were also recruited. The demographic characteristics of the two groups are compared in [Table/Fig-3].
Demographic characteristics of the study population.
| Rheumatoid Arthritis Patients | Healthy Volunteers |
|---|
| Age in yearsMean± SDRange | 38.45±10.3918 to 60 | 38.45±10.5421 to 60 |
| SexMalesFemales | 5 (16.13%)26 (83.87%) | 5 (16.13%)26 (83.87%) |
| TOTAL | 31 (100%) | 31 (100%) |
Out of the 31 RA patients studied, 19 (61.29%) were double seropositive, 11 (35.48%) single seropositive and 1 (3.23%) patient was negative for both RF and Anti-Cyclic Citrullinated Peptide (Anti-CCP). The duration of disease in patients ranged from 0 to 14 years with a mean of 3.57 years.
The mean CDAI score among RA patients was 11.26 with a standard deviation of 8.73. Patients were categorized into four groups on the basis of the CDAI score. There were four patients in remission while 14, eight and five patients had low, moderate and high disease activity, respectively.
AN, as assessed on the basis of the five cardiovascular reflex tests, revealed the following data- 80.65% of RA patients and 51.61% of the healthy subjects showed evidence of AN [Table/Fig-4]. A chi-square test result of 5.833 was obtained with a p-value of 0.016 (significant at p<0.05). Odds ratio was calculated to be 3.9063 with a 95% CI = 1.2545 to 12.1633. A comparison of the distribution of different grades of AN between the two groups is depicted in [Table/Fig-5,6].
Hundred percent stacked bar chart depicting prevalence of AN in RA patients and healthy volunteers.
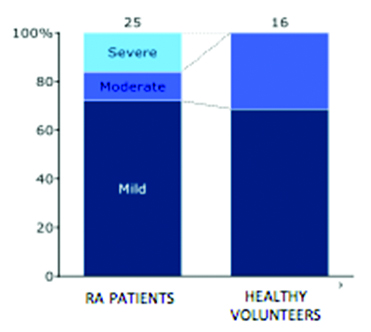
Proportion of different grades of AN in RA patients and healthy volunteers.
| Grade of Autonomic Neuropathy | Rheumatoid Arthritis Patients | Healthy Volunteers |
|---|
| Mild | 18 (72%) | 11 (68.75%) |
| Moderate | 3 (12%) | 5 (31.25%) |
| Severe | 4 (16%) | 0 (0%) |
| TOTAL | 25 | 16 |
Hundred percent stacked bar chart depicting proportion of different grades of AN in RA patients and healthy volunteers.
RA: Rheumatoid Arthritis
[Table/Fig-7] depicts the distribution of grades of AN in patients of RA with varying disease severity. AN was seen in 75% of RA patients in remission, 85.71% patients with low disease activity, 75% of patients with moderate disease activity and 80% of patients with high disease activity. No significant correlation was found between disease severity and AN with a Pearson’s correlation coefficient of 0.110 (p= 0.556).
Disease activity-wise distribution of AN.
| Disease Activity | Total Number | Grade of Autonomic Neuropathy | Number |
|---|
| Remission | 4 | None | 1 |
| Mild | 2 |
| Moderate | 0 |
| Severe | 1 |
| Total prevalence of Autonomic neuropathy | 3 (75%) |
| Low disease activity | 14 | None | 2 |
| Mild | 10 |
| Moderate | 1 |
| Severe | 1 |
| Total prevalence of autonomic neuropathy | 12 (85.71%) |
| Moderate disease activity | 8 | None | 2 |
| Mild | 5 |
| Moderate | 0 |
| Severe | 1 |
| Total prevalence of autonomic neuropathy | 6 (75%) |
| High disease activity | 5 | None | 1 |
| Mild | 1 |
| Moderate | 2 |
| Severe | 1 |
| Total prevalence of autonomic neuropathy | 4 (80%) |
[Table/Fig-8] compares autonomic dysfunction between the two genders in the case group. Autonomic dysfunction was found in 88.46% females, which is more than two times than in males (40%). However, the association could not be studied statistically due to the small sample size and low prevalence of RA in males.
Gender-wise distribution of AN in RA patients.
| Grade of Autonomic Neuropathy | Total | Overall Prevalence |
|---|
| Sex | None | Mild | Moderate | Severe |
|---|
| Female | 3 | 17 | 3 | 3 | 26 | 88.46% |
| Male | 3 | 1 | 0 | 1 | 5 | 40% |
Correlation coefficient for age and grade of AN is 0.031 (p=0.868), which implies no statistical significance.
The number of symptoms reported (SAS-A score) and the total impact score (SAS-B score) of the RA patients and healthy volunteers is compared in [Table/Fig-9]. Among the patients, both SAS-A and SAS-B scores were found to be significantly correlated with the disease severity (p-value of 0.001 and 0.004, respectively). However, there was no significant correlation with age, serological status and disease duration. Also, SAS scores did not correlate with the results obtained from grading of AN performed using the five reflex tests. (r=0.16, p=0.38 for SAS-A and r=0.166, p=0.372 for SAS-B).
Reporting of symptoms between RA patients and healthy volunteers.
| RheumatoidArthritis Patients | HealthyVolunteers | |
|---|
| Number of symptoms (SAS-A Score) | 2.45±2.00 | 1.45±1.46 | Student t-test = 2.252p=0.028 |
| Total impact score (SAS-B Score) | 6.19±6.05 | 3.68±4.09 | Student t-test = 1.919p=0.06 |
Discussion
Both previous and recent studies have confirmed the occurrence of extra-articular manifestations in RA [18-20]. In their study, Gabriel SE et al., concluded that the presence of extra-articular features was a strong predictor of mortality in RA patients [8].
This cross-sectional study, undertaken to assess the prevalence and severity of autonomic dysfunction in RA patients, examined in 31 patients and 31 healthy volunteers. Our study showed that 25 out of 31 (80.65%) RA patients and 16 out of 31 (51.61%) healthy volunteers had evidence of autonomic dysfunction. Thus, prevalence of AN was significantly more in RA patients than in the healthy volunteers (p=0.016, significant at <0.05). This rate observed in the case group is in concordance with the results obtained by Saraswathi PV et al., who used the three heart rate variability tests and found that 81.80% of 207 RA patients had deranged parasympathetic function [14]. However, this rate is much higher than that reported by Maule S et al., (15%), Louthrenoo W et al., (47%) and Toussirot E et al., (60%) [9,21,22]. This variation may be due to the difference in the criterion of abnormality. Toussirot E et al., considered AN present if a minimum of two tests were abnormal [22], while we considered even a single abnormal test as evidence of AN.
An unexpected finding in the present study was that more than half (51.61%) of the 31 healthy volunteers had some level of AN. This may be due to use of a single abnormal test as evidence of AN. An interesting observation was that out of the 16 healthy volunteers who tested positive, 13 had a deranged heart rate response to standing. Furthermore, in eight healthy subjects, this was the only abnormal test. Even among RA patients, this was the test that was most commonly reported abnormal. A possible explanation for this could be the particular sequence in which the five tests were performed. Abawajy J et al., recommend using biochemistry features to enhance the accuracy of the Ewing battery of tests [23]. Holter-monitor algorithm analysis could also serve as a more sensitive and specific method of testing heart rate variability.
In both the groups, mild autonomic dysfunction was most common-72% in RA patients and 68.75% in healthy subjects. However, while four RA patients had severe AN, none of the healthy volunteers showed presence of severe AN. These findings support the hypothesis that, as compared to the general population, the pathogenic processes responsible for AN are more active in patients of RA, and even more so in severe RA. Vasculitis, amyloid deposition and autoantibodies to ANS structures have been proposed, but the exact mechanisms are yet to be clearly understood.
Results from the present study did not show any significant correlation between grade of AN and age (p=0.868), serological status (p=0.7), disease duration (p=0.54) or disease severity (p=0.556). This is in agreement with some previous studies. Louthrenoo W et al., failed to demonstrate association with duration of disease, swollen joint count, ESR, Ritchie Articular Index and RF titres [21]. Similarly, the study by Saraswathi PV et al., showed no correlation with age, rheumatoid factor status or disease duration [14]. On the other hand, a number of studies have reported significant correlation between AN and disease factors, especially disease severity. Yadav R K et al., and Anichkov DA et al., independently found that heart rate variability was significantly altered in RA patients and was associated with disease activity [24,25]. A tendency of greater cardiac AN with positive rheumatoid factor status and higher C-Reactive Protein (CRP) levels has also been reported [26-28].
In the Indian population, decreased heart rate variability, indicating CAN, and its correlation with disease activity has been observed [24]. Recent reports by Syngle et al., have found that different therapies including Disease Modifying Anti-Rheumatic Drugs (DMARDs) and drugs targeting the interleukin pathway have the potential to improve AN [13].
The number of autonomic complaints obtained from the SAS questionnaire administered was markedly higher in RA patients (p=0.028) as compared to the healthy volunteers and was significantly correlated with severity of disease (p=0.001). The results were, however, divergent from those obtained by the five cardiovascular tests. This could be due to the subjective nature of the SAS questionnaire as opposed to the objective cardiovascular reflex tests. Patients with higher disease activity may tend to over report their symptoms. Though open to bias, the SAS questionnaire serves as a subjective means of assessing the quality of life of RA patients. It determines the patient reported variables in disease activity, treatment adherence, satisfaction and the psychosomatic co-morbidities.
Limitation
Shortcomings of the study include its small sample size. The measures of AN used, namely the cardiovascular reflex tests and the SAS scores are semi-quantitative at best. AN may be a part of subclinical neuropathy, as an extension of RA vasculitis. We did not check for clinical or electrophysiological evidence of the high background prevalence of AN, found even among the healthy population. This could be due to unexplained methodological issues and may falsely decrease the relevance of the obtained results. Further, the study is not prospective in nature, which is required to analyze the future implications of AN on morbidity and mortality.
Conclusion
Upon carrying out the study and subsequent analysis, the null hypothesis was rejected and the prevalence of AN measured both subjectively and objectively is significantly higher in patients of RA as compared to healthy volunteers. No statistically significant association was observed with the patient’s age, disease duration and serological status in both measures. As autonomic dysfunction has been linked to a reduced quality of life and serious and potentially life-threatening cardiovascular complications, further research must be carried out to get a better understanding of the condition. Longitudinal follow up studies involving larger number of patients will help in getting a clearer picture of the pathogenesis, influences and long-term effects of AN in patients of RA.