The term ‘Teratoma’ is derived from a greek word “teraton” which means monster. It was named so because these tumours contain elements derived from all the 3 germ cell layers of the ovary. MCT are the most common germ cell tumours of the ovary accounting for 10-20% of all the ovarian tumours. They comprise approximately one third of all the benign ovarian neoplasms and are most common ovarian tumour in patients between 20 to 40 years of age [1]. Torsion, rupture and infection are more common complications of MCT [2,3] and only very rarely do these tumours undergo a malignant transformation. The possibility of such malignant transformation must be kept in mind when either the patient is over 45 years of age or the tumour diameter is more than 10 cm. [4]. Although there are several studies of MCT in Asian countries, there are only very few retrospective cross sectional studies from India to understand the epidemiology of the disease [5]. Since the prognosis for malignant transformation of MCT is very poor with a five year survival of only 15-30%, it is essential to diagnose and treat them early in the course of disease [6]. We retrospectively analysed the clinicopathological characteristics and the trends of all the teratomas of the ovary at our institution over a 25-year study period.
Materials and Methods
This was a retrospective study of all the teratomas of the ovary received in the Department of Pathology at a tertiary care hospital, India. It included a total of 242 cases of ovarian teratomas over a period of 25 years from 1990 to 2014. The data regarding age, size, laterality, gross, morphological features, complications and surgery performed was retrieved from the pathological archives and analysed using SPSS software version 22. Descriptive statistics was used and the results were expressed as percentages.
Results
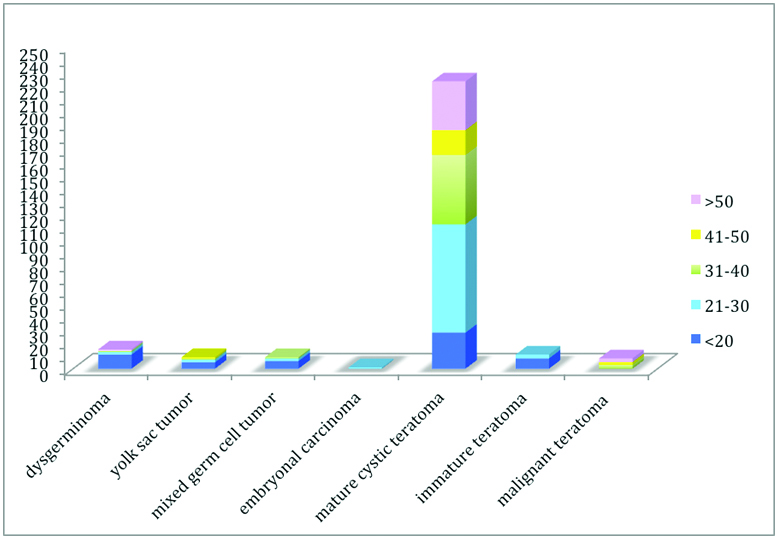
A total of 1102 ovarian neoplasms were received in our department during a period of 25 years from 1990 to 2014. 25.7% (284/1102) of all the ovarian tumours so received in our department were Germ cell tumours. Of the consecutive 242 cases of ovarian teratoma 223 were MCT. These were the most common tumours in our study forming 20.2% of the total ovarian neoplasms and 78.5% of all the germ cell tumours. [Table/Fig-1] represents the age wise distribution of various germ cell neoplasms found in our study. As is evident from the histogram, MCT was most common tumour in all the age groups. Apart from MCT there were 11 cases of immature teratoma and 8 cases of malignant teratoma each. The rate of malignant transformation of mature cystic teratoma was 3.5% in this study.
Age wise distribution of various germ cell neoplasms in our study.
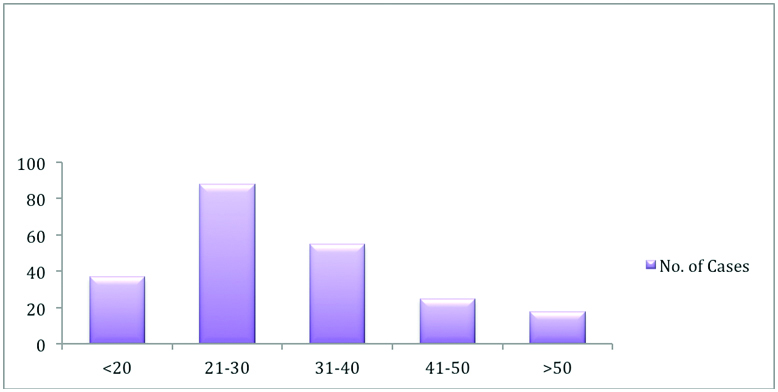
The mean age of the patients with MCT was 32.5 ± 13.11 (mean ± standard deviation) years ranging from 8 years to 70 years. The median age was 30 years. [Table/Fig-2] represents the age wise distribution of all the MCT included in our study. It was seen that maximum number of cases i.e., 39.4% were in the age group of 21–40 years and only 8% of patients presented beyond 50 years of age. It was noted that although the tumour incidence was common in reproductive age group, 25% of patients were also in the perimenopausal and postmenopausal age group.
Age wise distribution of all the mature cystic teratoma included in our study.
[Table/Fig-3] represents the common clinical presentation of our patients. Lump abdomen was the most common presenting complaint in our study followed by pain abdomen. A 7.6% of the cases presented with acute pain abdomen due to torsion or rupture of these tumours. While 10% of the patients were asymptomatic, 15.8% were diagnosed incidentally either during pregnancy work up, intraoperatively or during emergency cesarean section operations. Among those diagnosed during pregnancy 30% were diagnosed during the first trimester itself.
Clinical presentation of patients.
| Presenting Complaint | Percentage of cases |
|---|
| Pain | 23.5 |
| Lump | 33.7 |
| Cyst | 7.3 |
| Asymptomatic | 10.1 |
| Coincidental | 15.8 |
| Polymennorrhea | 4.4 |
| Infertility | 4.8 |
The complications of MCT were seen in total 32 cases which included torsion, rupture and malignant transformation [Table/Fig-4]. Torsion was the most common complication among all the other complications. It was seen that MCT was also the most common tumour to undergo torsion among the various ovarian tumours [Table/Fig-5]. Malignant transformation (MT) of this tumour though rare was seen in 3.5% (8/223) of the cases. Squamous Cell Carcinoma (SCC) was the most frequent MT seen in four out of eight cases (50%), followed by transitional cell carcinoma, adenocarcinoma and malignant melanoma. It was seen that 62.5% (5/8) of the patients developing MT were above 40 years of age. It was also noted that in 87.5% (7/8) of cases the size of the tumour developing this transformation was more than 10 cm. However, when the laterality was compared, equal number of patients developed, malignant transformation on left as well as right side.
Complications of mature cystic teratoma in our study.
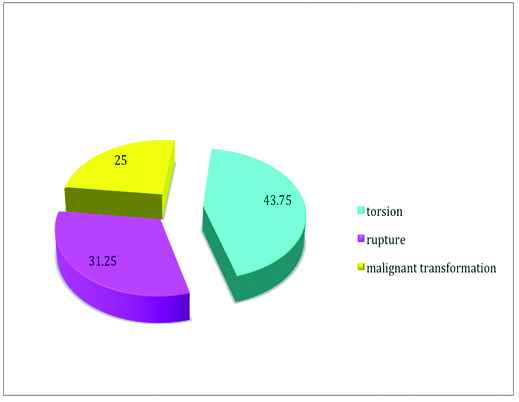
Graphical representation of various tumours undergoing torsion in our study.
Gross examination
It was seen that while 50.3% of the tumours were right sided, 40.8% were left sided and the remaining 8.9% were bilateral in nature. The mean size of tumour was 8.6±3.1 cm ranging in size from 1.5 cm to 17 cm. The median size of MCT in our study was 8 cm. 73.9% of the tumours in our study were <10 cm in size.
Most of the tumours were either (56.9%) solid cystic or cystic on cut surface. There was no case of solid teratoma in our study. The variegated and haemorrhagic cut surface was seen in cases associated with torsion or rupture of these tumours. These solid cystic tumours were filled with a cheesy material in 89% cases but the presence of elements like teeth; bone and hair were also seen in some of them [Table/Fig-6].
Gross examination findings of the patients.
| GROSS | | |
|---|
| LATERALITY | No. Of Cases | Percentage of cases |
|---|
| Right | 112 | 50.3 |
| Left | 91 | 40.8 |
| Bilateral | 20 | 8.9 |
| SIZE (cm) | | |
| <10 | 165 | 73.9 |
| 11 TO 20 | 57 | 27.5 |
| 21 TO 30 | 1 | |
| CUT SECTION | | |
| Cystic | 66 | 29.6 |
| Solid/Cystic | 127 | 56.9 |
| Variegated | 12 | 5.3 |
| Haemorrhagic | 18 | 8.1 |
| CONTENT | | |
| Cheesy material | 199 | 89.2 |
| Bone and Teeth | 10 | 4.4 |
| Serous fluid | 8 | 3.6 |
| Mucinous fluid | 6 | 2.7 |
Microscopic examination
Histopathological examination revealed that all of the mature teratomas consisted of tissues derived from ectoderm and mesoderm. The endodermal derivatives were seen in 26% cases. Though, 6 cases of struma ovarii were also seen, these patients did not show any signs of hyperthyroidism [Table/Fig-7].
Morphological types of tissue found in our study.
| Types of ectodermal tissue | Cases |
|---|
| Squamous epithelium | 204 |
| Skin and appemndage | 191 |
| Keratin | 156 |
| Neural | 5 |
| Choroid epithelium | 2 |
| Transitional epithelium | 6 |
| Types of Mesodermal Tissue | Cases |
| Blood vessel | 223 |
| Bone | 8 |
| Cartilage | 25 |
| Fibroadipose tissue | 215 |
| Hematopoeitic tissue | 17 |
| Lymphatic | 55 |
| Myxoid | 23 |
| Skeletal muscle | 47 |
| Smooth muscle | 189 |
| Teeth | 10 |
| Types of endodermal tissue | Cases |
| Thyroid | 6 |
| Respiratory epithelium | 87 |
| Intestine | 96 |
| Glands | 128 |
The ectodermal tissues consisted of skin with adnexa in almost all the cases, followed by keratin material, Nervous tissue and nerve fibers, transitional epithelium and least commonly choroid epithelium. Mesodermal derivatives included blood vessels, hematopoietic tissue, spindle shaped cells, adipose tissue, fibromuscular tissue, cartilage, skeletal muscle fibers, smooth muscle fibers, fibrocollagenous tissue, myxoid tissue and lymphatic. The endodermal derivative in the form of thyroid gland, respiratory epithelium and gastrointestinal tract Epithelium was seen in 84.7% cases (excluding glands). Endodermal derivatives appeared in the form of glands and ducts, thyroid tissue, respiratory and intestinal epithelium [Table/Fig-7]. Fifteen cases of epidermal cyst and 46 cases of benign simple cyst found were excluded from the final analysis.
Treatment
The treatment consisted of cystectomy in 48% of the cases, unilateral salpingoophrectomy in 30%, hysterectomy with bilateral salpingoophrectomy in 20% of cases. Remaining 2% of cases were treated with only bilateral salpingoophrectomy.
Discussion
Mature cystic teratomas (dermoid cysts) are one of the most common benign ovarian neoplasms, accounting for 10 to 20% of all ovarian tumours [7]. Teratomas may occur at any age but the peak incidence is reported between 20 and 40 years of age [1]. In this study we found that the 20% of all the ovarian tumours over the period of 25 years were mature teratoma. In accordance with the previous studies the average age of patients in our study was 32.5 ± 13.11 with maximum cases in the reproductive age group (20-40 years). Since they are common in reproductive age group, it is essential to diagnose them early in life as conservative ovarian surgery done early has minimal impact on puberty and fertility [8]. It is hypothesized that they are found in this age group because of their development from a single primordial germ cell, which has completed meiosis I and where meiosis II is suppressed [9].
In our study 50.3% of teratomas were located on the right side, while 40.8% were on the left side and the remaining was bilateral. This was in contrast to Mumtaz et al., who found 64% cases on left side [10]. However, Ismail RS and Chun et al., found 72.2% and 55.4% of benign teratomas on right side respectively [11,12]. Bilateral teratomas are uncommon with the reported rate of 8.2 to 16.7% [13,14], which is consistent with our study.
The mean size of tumour was 8.6 ± 3.1 cm, ranging in size from 1.5 cm to 17 cm with 73.9% of tumours being > 10cm in diameter. This is in accordance with the other studies where 60% of tumours were 5-10 cm in diameter [11,13,15,16].
Patients of teratoma develop symptoms like pain, menstrual irregularities, urinary and gastrointestinal complaints due to the size of these tumours [13,17]. The most common symptom of these tumours was pain abdomen in the previous studies; however, in our series maximum number of patients (37%) presented with lump abdomen followed by pain abdomen in 25% of them. MCTs are known for being discovered as an incidental finding during a routine physical examination or during pelvic surgery for different reasons quite often, frequency of asymptomatic patients varies from 16.6 to 75.5% [17-22] in various studies. In our study, only 10% of patients were asymptomatic and 17% presented as a coincidental finding during antenatal period or caesarian section.
In our study, mature teratomas were complicated by torsion, rupture and malignant transformation. Torsion of the pedicle was the most common complication of these tumours with the frequency of 3.5 to 9.2% [13,14,18,19] in consistence with our study, where it was seen in 6.2% of the patients. However, in contrast to Kandasamy Vijayalakshmi et al., who reported mucinous cystadenoma to be the most common ovarian tumour undergoing torsion, our study reported MCT to be the same [23].
Malignant transformation of these tumours is a very rare complication occurring in 1 to 3% of patients, most often in the form of a squamous cell carcinoma [18,19,24]. Although, the most common malignancy to arise in our study was also squamous cell carcinoma, the incidence of malignant transformation was a bit higher than the reported literature accounting upto 3.5%. This relatively high incidence may be attributable to the fact that ours is a tertiary care center in a major metropolitan city and most of the patients with advanced diseases are referred to us from the various primary and secondary health care centers located in the two adjoining states including ours. Consistent with the previous studies, the most important prognostic factors for malignant transformation of mature teratomas appeared to be age and tumour diameter in this study [9,25]. Even thought, mature cystic teratomas occurred mostly in the reproductive age group, malignant transformation was more common in the post menopausal women [22].
Grossly, we found that all the tumours included in our study were either cystic or solid cystic which is consistent with the study conducted by Morillo et al., who found it in 96% cases [26]. But, unlike their study, we did not get any case of solid teratoma so far. The WHO classification of ovarian tumours (2003) has separated epidermoid cysts from dermoid cysts [27]. This is because epidermoid cysts are considered to arise from squamous metaplasia of epithelial elements and are lined by stratified squamous epithelium, without evidence of mesodermal or endodermal tissue. However, only few cases of epidermoid cyst encountered during re-evaluation were not included in this study.
In our study, where skin and its appendages were the most frequent ectodermal derivatives, each constituting 95.6%, similar to other studies, it was observed in 100% of the tumours in a case series reported by Ayan et al., [21]. The mesodermal structures in the form of bone and/or cartilage either or both were detected in 8% and 15% of our cases [14]. In contrast, a much higher percentage of MCTs having bone and cartilage such as 22.9% bone and 18.7% cartilage reported by Ong HC et al., 19.1% bone and 39% cartilage in series of Sah et al., were reported by other studies [17,28].
Limitation
Although, the duration of the study was long, it was conducted in only one tertiary care centre of India. Moreover, there are only few studies conducted on this topic from other parts of India. Hence, we recommend that more studies in the form of meta-analysis must be done to assess the exact burden of the disease.
Conclusion
MCT are not only the most common germ cell tumours of the ovary but are also the most common tumours of the ovary itself. Though predominantly seen in reproductive age group they may also be seen in children and postmenopausal women. Mostly, the patients present with lump abdomen. Torsion is the most common complication of this tumour. Larger tumours are more prone to undergo torsion. Age of the patient and the size of the tumour are important factors in terms of malignant transformation of teratomas. Since 8.9% of these tumours were bilateral, it is essential to thoroughly examine the contralateral ovary to rule out this possibility. Early diagnosis and early treatment in terms of a conservative surgical approach may have a greater positive impact on the puberty and fertility of these patients.