Primary Pulmonary Artery Intimal Sarcoma Case with Elevated Coagulation Markers
Koshiro Sakai1, Yoshino Minoura2, Taiju Matsui3, Kyoichi Kaneko4, Yoichi Kobayashi5
1 Senior Resident, Division of Cardiology, Department of Medicine, Showa University School of Medicine, Shinagawaku, Tokyo, Japan.
2 Assistant Professor, Division of Cardiology, Department of Medicine, Showa University School of Medicine, Shinagawaku, Tokyo, Japan.
3 Assistant Professor, Division of Cardiology, Department of Medicine, Showa University School of Medicine, Shinagawaku, Tokyo, Japan.
4 Assistant Professor, Division of Cardiology, Department of Medicine, Showa University School of Medicine, Shinagawaku, Tokyo, Japan.
5 Professor, Division of Cardiology, Department of Medicine, Showa University School of Medicine, Shinagawaku, Tokyo, Japan.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Yoshino Minoura, 1-5-8 Hatanodai, Shinagawaku, Tokyo-142-8666, Japan.
E-mail: yoshinomm@med.showa-u.ac.jp
Primary Sarcoma of the Pulmonary Artery (PAS) is a very rare and miserable disease. The clinical signs and symptoms of PAS are non- specific, which usually prevents diagnosis before surgery or autopsy. The current guidelines for the diagnosis and treatment of PAS have not been well established. Several reported cases of PAS have been mistaken for Pulmonary Artery Thromboembolism (PTE), because the clinical signs and symptoms of PAS are non-specific. Elevated coagulation markers are generally absent in PAS and therefore, support a differential diagnosis of PTE. We herein report a patient with PAS who presented with elevated coagulation markers and later showed rapidly improved values mimicking response of PTE to anticoagulant therapy.
D-dimer, Hypercoagulability, Super-hyperfibrinolysis
Case Report
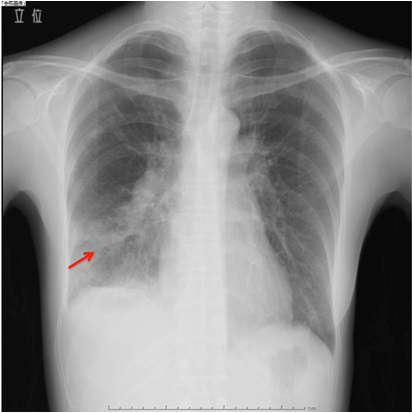
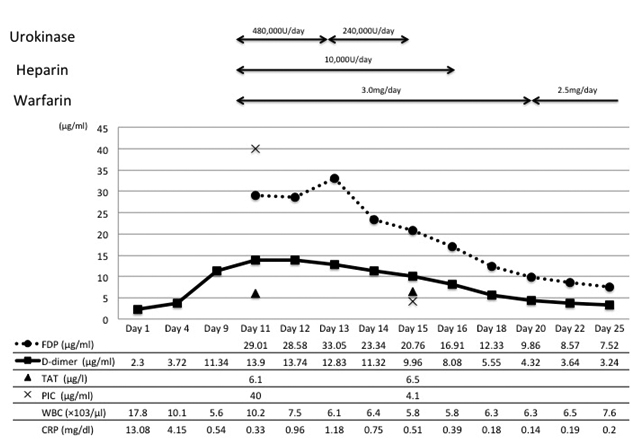
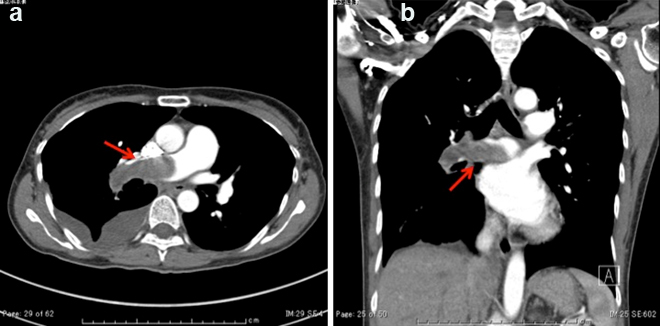
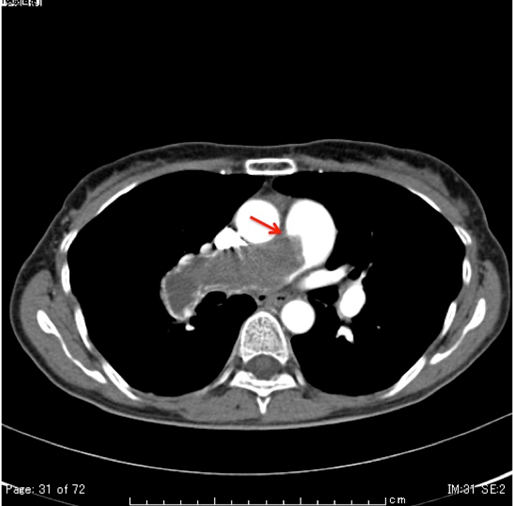
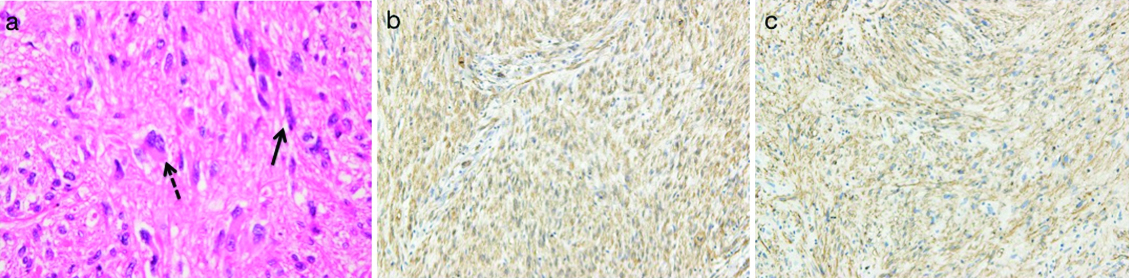
A 49-year-old woman was admitted to the Department of Respiratory Medicine with fever and productive cough that had lasted for two weeks. Her cough had progressively worsened even though she had received a seven day course of empirical antibiotic therapy (garenoxacin 400 mg/day, pazufloxacin 1000 mg/day and clindamycin 1200 mg/day) under a diagnosis of pneumonia at a local hospital. A chest X-ray revealed a wedge-shaped shadow in the right lower lung field [Table/Fig-1]. No signs of acute distress were observed in the physical examination on admittance. Her vital signs were within normal range. Blood tests revealed inflammatory activity (WBC 17800/ml, CRP 13 mg/dl) and a slight increase of D-dimer (2.3 μg/ml). An echocardiograph demonstrated a slight right ventricular overload. After admission to the Department of Respiratory Medicine, she was switched to a course of stronger antibiotics (meropenem 3 g/day and levofloxacin 500 mg/day) as per the antibiotics sensitivity test. On the 11th day at hospital her symptoms and signs showed exacerbation and her D-dimer level (normal range <1.0 μg/ml) spiked to 13.90 μg/ml. Similarly sudden increase were observed in other coagulation markers [Thrombin-Antithrombin III (TAT) complex-6.1 μg/l (normal range 1.0-4.1 μg/l); Prothrombin Fragment 1+2 (PTF 1+2)-399 pmol/l (normal range 69-229 pmol/l); Fibrin Degradation Product (FDP)-29.01 μg/ml (normal range <5.0 μg/ml)], and in the fibrinolysis marker [Plasmin-α2 Plasmin Inhibitor Complex (PIC)- ≥40 μg/ml (normal range ≤0.8μg/ml)] [Table/Fig-2]. Hypercoagulability and super-hyperfibrinolysis were both observed. A contrast-enhanced spiral CT of chest showed massive filling defects in the right pulmonary artery and pneumonia of the right lower lung [Table/Fig-3]. We initially suspected PTE and started the patient on thrombolytic and anticoagulation therapy: heparin 10000 U/day, warfarin 3 mg/day, and urokinase 480000 IU/day (11th and 12th day at hospital) and 240000 IU/day (13th and 14th day at hospital). The ventilation-perfusion scintigraphy showed a reduced perfusion of the right field and no defect of ventilation. On the 15th hospital day, the coagulation system marker was unchanged (TAT 6.5 μg/l) but the fibrinolytic system marker was reduced (PIC-4.1 μg/ml). The FDP-D-dimer values were sharply down on the same day (FDP-20.7 μg/ml, D-dimer-9.9 μg/ml) [Table/Fig-2]. Subjective symptoms and inflammatory markers were improved (WBC-5800/ml, CRP-0.3 mg/dl). No uptake in the pulmonary artery was found in Gallium (Ga-67) scintigraphy. No tumour markers were significantly elevated in laboratory blood tests. As a next step we suggested that she undergo PET to rule out malignant disease more precisely, but the patient refused for the PET. She was discharged on the 25th hospital day with a D-dimer level of 3.24 μg/ml under warfarin (2.5 mg/day) treatment. We scheduled the regular inspection for her as an outpatient. At about 3 months after discharge her D-dimer level had not recovered to the normal range (2.6 μg/ml) but her coagulation and hyperfibrinolysis system markers had dropped below the levels measured at discharge (TAT 4.2 μg/l, PIC 1.2 μg/ml). Massive filling defects in the pulmonary artery main trunk were somewhat increased in a follow up enhanced CT of chest [Table/Fig-3,4]. PET showed an accumulation of 18F-fluorodeoxy glucose in the center of the same pulmonary artery lesion [Table/Fig-5]. A Dynamic Contrast Enhanced MRI (DCE-MRI) depicted an expansion of the massive filling defects compared to the findings shown on the enhanced CT [Table/Fig-6]. For diagnostic and curative treatment, the patient underwent a surgical resection of the mass within the lumen of the pulmonary artery and right lung. Based on the histopathological and immunohistochemical findings, the tumor was as an intimal sarcoma with myofibroblastic differentiation [Table/Fig-7]. After the PAS resection she received radiation therapy (total 60Gy). She survived for 20 months after her first admission.
The chest X-ray on admission to our hospital shows a wedge-shaped shadow in the right lower lung (red arrow).
Clinical courses. The anticoagulation therapeutic agents are shown at the top of the graph. The blood test markers also are presented: FDP, fibrin degradation product; D-dimer; TAT, thrombin-antithrombin III complex; PIC, plasmin-α2 plasmin inhibitor complex; WBC, white blood cell; CRP, C-reactive protein.
(a,b) A contrast-enhanced spiral CT chest on the 11th hospital day shows massive filling defects in the right pulmonary artery and pneumonia of right lower lung (red arrow).
A contrast-enhanced chest spiral CT after three months. The massive filling defects expand into the main trunk of the pulmonary artery compared with those at admission (red arrow).
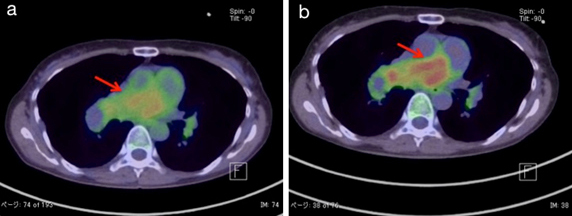
Positron Emission Tomography (PET) after three months. PET shows an accumulation of 18F-fluorodeoxy glucose in the center of the same pulmonary artery lesion in both (a) An early scan; (b) delayed scan. The SUV max values of the scans are (a) 5.86 and (b) 9.73 (red arrow).
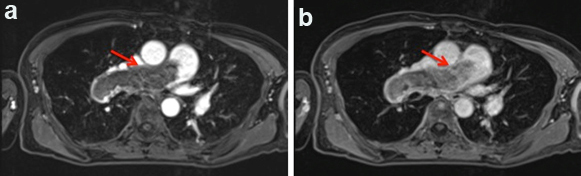
Dynamic Contrast Enhanced MRI (DCE-MRI) after three months; (a) The early phase; (b) The delayed phase (red arrow).
(a) Hematoxylin-Eosin, spindle cells with heteromorphic nuclei (solid arrow) and giant cells (dotted arrow); (b) Alpha-smooth muscle actin positive; (c) CD34 positive.
Discussion
PAS is a very rare and miserable disease [1]. PAS presents with symptoms and findings suggestive of PTE. Wackers FJ et al., reported that PAS should be suspected when a patient presents with haemoptysis [2]. Other reports suggested symptoms of weight loss, fever, and digital clubbing for the diagnosis of PAS [3]. The most important points are abnormalities of the coagulation fibrinolysis system. Guo W et al., reported that D-dimer values were within the normal range in all the nine PAS cases [4]. Several case reports also suggested that coagulation fibrinolysis system markers in normal range is a point to suspect PAS [5,6]. Yamamoto K et al., suggested that anticoagulation and thrombolytic therapy can not improve the reduction of pulmonary artery massive filling defects by imaging study in case with PAS [3]. In our knowledge, there are few PAS cases with the elevation of coagulation and fibrinolysis markers [4]. In this case, the D-dimer value in our present patient soared to 13.90 μg/ml on the 10th hospital day but then recovered to 3.24 μg/ml within two weeks after the commencement of anticoagulation and thrombolytic therapy. Several processes and conditions could have led to the elevated coagulation markers in our patient. First, the markers could have been raised by inflammation associated with pneumonia. Yet the initial inflammatory reactions in our patient were controlled by day 10 (CRP-0.3 mg/dl). A shadow was still be evident on X-ray at that point, but the inflammatory activity was apparently too weak to actively affect the coagulation system. Second, we hypothesised that the PAS was surrounded by a thrombus at that point. The D-dimer would have presumably decreased in parallel if the thrombus had been rapidly dissolved by the anticoagulation therapy. This remains a speculation, and we have no other explanations for the discrepancy between the inflammatory markers and coagulation markers. If we had operated when the patient’s D-dimer spiked to 13 μg/ml, we might have detected a thrombus surrounding the PAS. In addition to contrast CT and Gallium (Ga-67) scintigraphy, contrast MRI and PET have recently been reported as useful imaging modalities [7]. The chest enhanced CT of our patient showed no improvement of the massive filling defects at three months after anticoagulant therapy, though her elevated coagulation markers did not recur during the follow-up. PAS should be considered as a diagnosis when the imaging findings are inconsistent with the coagulation marker response to anticoagulant therapy. Given the importance of an early diagnosis of PAS for the patient’s prognosis, we recommend that PAS be diagnosed based on overall findings rather than coagulation markers.
Conclusion
It has been reported that the elevation of coagulation markers is useful for differential diagnosis between PAS and PTE. However, this case suggests considering the possibility of PAS even when coagulation markers are high.
[1]. Nakahira A, Ogino H, Sasaki H, Katakami N, Long-term survival of a pulmonary artery sarcoma produced by aggressive surgical resection and adjuvant chemoradiotherapyEuropean Journal of Cardio-Thoracic Surgery 2007 32:388-90. [Google Scholar]
[2]. Wackers FJ, Van Der Schoot JB, Hampe JF, Sarcoma of the pulmonary trunk associated with hemorrhagic tendency. A case report and review of the literatureCancer 1969 23:339-51. [Google Scholar]
[3]. Yamamoto K, Nozue T, Tsuchida M, Iwaki T, Nagamine H, Yasuda T, Pulmonary embolism caused by intimal sarcoma of the pulmonary arteryInternal Medicine 2012 51:3031-34. [Google Scholar]
[4]. Guo W, Zhang W, Huang X, Liang Y, Gan H, Chen D, Clinical characteristics of 9 patients with pulmonary artery sarcomaZhonghua Xin Xue Guan Bing Za Zhi 2014 42:38-42. [Google Scholar]
[5]. Dornas AP, Campos FT, Rezende CJ, Ribeiro CA, Amaral NF, Correa Rde A, Intimal sarcoma of the pulmonary artery: A differential diagnosis of chronic pulmonary thromboembolismJ Bras Pneumol 2009 35(8):814-18. [Google Scholar]
[6]. Blackmon SH, Rice DC, Correa AM, Mehran R, Putnam JB, Smythe WR, Management of primary pulmonary artery sarcomasThe Annals of Thoracic Surgery 2009 87:977-84. [Google Scholar]
[7]. Ito K, Kubota K, Morooka M, Shida Y, Hasuo K, Endo H, Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolismAnnals of Nuclear Medicine 2009 23:671-76. [Google Scholar]