Carcinoma of uterine cervix is most common gynaecological malignancy in our country and most of the patients presents in locally advanced stage such as Federation of Gynaecology and Obstretics (FIGO) stage II and III [1]. Radiotherapy plays an important role in treatment of carcinoma of uterine cervix and combination of EBRT and ICBT is accepted as definitive mode of treatment. ICBT has been considered as an essential and integral component to maximize the curative potential of radiotherapy in uterine cervix cancer management [2]. The traditional practice of Low Dose Rate (LDR) ICBT was replaced by HDR ICBT in most of the countries of the world [3]. Though, some practical gains such as shorter treatment time, less radiation hazard to health care givers, by HDR ICBT, still there are concerns about late toxicity of large dose per fraction [4]. In treatment of cancer cervix ICBT with HDR brachytherpay is being performed since many decades, but there is no consensus about an optimal fractionation schedule available in English literature. American Brachytherapy Society (ABS) recommends that individual fraction size should be less than 7.5 Gy per fraction and number of fractions should range from four to eight depending on fraction size [2].
We are practicing HDR-ICBT schedule of 6 Gy per fraction per week for 4 fractions in our institute. So, this prospective study was conducted with the aim to assess the feasibility and tolerability between two different dose fractionations of HDR-ICBT such as 6 Gy per fraction per week for 4 fractions and 8 Gy per fraction per week for 3 fractions in terms of local control rate, disease free survival, and failure pattern, early and late toxicity.
Materials and Methods
This is a prospective randomized control study conducted in Department of Radiotherapy, Sri Venkateswara Institute of Medical Sciences, Tirupati, a tertiary care center in Southern India. From March 2013 to August 2014, all patients diagnosed with carcinoma of uterine cervix were screened for inclusion in the study. The criteria for inclusion and exclusion of the patients followed in this study were shown in [Table/Fig-1]. All patients underwent complete evaluation by history taking, gynaecological examination and systemic examination. Histological proof of malignancy was attained through biopsy in all patients. All patients were evaluated by complete blood count, liver function tests, renal function tests, chest radiograph and, ultrasound abdomen and pelvis. CECT abdomen and pelvis or MRI pelvis was done as appropriate. FIGO Staging system [5] was used to stage the patients. This study was started after getting approval from institutional ethical committee. Written informed consent was taken from the patients before start of treatment.
Inclusion and exclusion criteria.
| Inclusion criteria: | Exclusion criteria: |
|---|
| i) Histo-pathologically confirmed carcinoma of uterine cervix cases. | i) FIGO stage IVA with Vesico-vaginal or recto-vaginal fistula and IV B cervical cancer. |
| ii) FIGO stage IB2 to stage IVA | ii) Previous history of pelvic irradiation |
| iii) Eastern Cooperative Oncology Group (ECOG) performance score of 0 to 2 | iii) Post hysterectomy carcinoma cervix cases |
| iv) Patients who has given approved informed consent | iv) Pregnant women or lactating mothers |
| v) Patients not completed planned treatment schedules. |
FIGO- International Federation of Gynaecology and Obstetrics
Treatment Allocation: All enrolled patients in this study received EBRT to pelvis with a dose of 46 Gy in 23 fractions at the rate of 2 Gy per fraction with or without concurrent weekly cisplatin chemotherapy at a dose of 40 mg/m2 as per institutional protocol followed by HDR-ICBT. Patients were assessed for ICBT after 15 fractions of EBRT and were applied whenever patients were fit for application during the course of EBRT and patients were allocated into two Arms by randomization done by the computerized random number tables. Patients in Arm A received the dose fractionation of 6 Gy per fraction per week, for 4 fractions, 1 week apart which was used as a control arm and patients in study Arm B received the dose of 8 Gy per fraction per week for 3 fractions, 1 week apart was the test arm.
EBRT was delivered to whole pelvis using linear accelerator (Elektra precise) machine using 15 MV photons. Radiation treatment techniques were customized as appropriate to the patients according to the extent of disease. For all patients, verification of the pelvic treatment fields was done by Electronic Portal Imaging Device (EPID) port film on treatment couch. The target volume included was primary tumour, lymph nodes and sub clinical disease. Patients were planned in either two parallel opposed Antero-Posterior-Postero Anterior (AP-PA) field or four-field box techniques or 3D conformal radiotherapy planning as indicated. All patients were planned in supine position. Portals were marked based on bony anatomy. The superior border for the AP-PA field was kept at L4 to L5 interspace and inferior border at the inferior border of obturator foramen or lower depending on disease extension to vagina to cover the tumour with a margin of 2 to 3 cm. The lateral border was kept 1.5 to 2 cm away from the lateral pelvic brim to include the pelvic nodes. For lateral fields, the superior and inferior borders were same as AP-PA field. The anterior border was kept just in front of the pubic symphysis and posterior border was set to cover the entire sacral hollow. Dose was delivered to centre of field with isocentric technique.
HDR-ICBT: Patients were assessed for ICBT fitness after completion of 15 fractions of EBRT. Applicator insertion was done when the uterine os was able to be sounded. Time period between each application was one week. Modified Fletcher Suit applicators–intrauterine tandem and paired ovoids of different sizes were used according to individual patient’s anatomy.
Procedure was done under strict aseptic conditions under conscious sedation. Patients were placed in lithotomy position. A Foley’s catheter was inserted and the balloon was inflated with 7 cc of iohexol contrast for identification of the bladder point. Cervical os was located, dilated with Hegar’s dilator. Then, length of uterine canal was measured with sound and appropriate intrauterine tandem was inserted. Two ovoids were selected based on individual anatomy were applied. Adequate posterior and anterior vaginal packing was done by regular betadine soaked gauze packs to push the bladder and rectum away and to stabilize the applicator. A rectal guide wire was inserted in to rectum to visualize the rectal points.
Then, patients were transferred to CT simulation room where CT pelvis was taken in treatment position and images sent to VARIAN BRACHY-VISION computerized treatment planning system in which dosimetric planning was performed. Point A was located at 2 cm lateral to the centre of uterine canal and 2 cm vertically above from top of uterine cervical flange (stopper) in the plain of uterine cavity. Total dose of 24 Gy was prescribed to Point –A in either 8 Gy per fraction x 3 fractions or 6 Gy per fraction x 4 fractions. The doses of urinary bladder and rectum were calculated on bladder and rectal reference points. The optimization of ICBT isodose curves was done by adjusting stepping source at 5mm dwell times in tandem and ovoids. ICRU (Report 38) [6] dose recommendations were followed to accept plans and doses to rectum and bladder were kept below than 80% of the prescribed point A dose, unless compromised. Dose of HDR- ICBT was delivered by using Varian Gamma Med plus remote after loading system with Ir192 isotope. For all patients treatment was delivered in supine position.
Follow up: Patients were assessed for tumour response after 6 weeks of completion of radiotherapy. Response assessment was done based on RECIST (Response Evaluation Criteria In Solid Tumours) criteria [7]. Patients were examined once in a month for first 3 months, then 3 monthly thereafter for one year and four monthly in second year. During follow up patients were evaluated for local recurrence, distant failures, rectal and bladder toxicity. All patients underwent thorough clinical examination and necessary investigations were done as per indications. Bladder and rectal morbidity was documented during follow up period and grading of late rectal and bladder morbidity done according to the Radiation Therapy Oncology Group/ criteria [8].
Statistical Analysis
Local pelvic control rate and disease-free survival were calculated from the date of start of treatment to the date of local or distant recurrence. Treatment failures were categorized as local pelvic recurrence (cervix, vagina or pelvic nodes) or distant recurrence (metastases to lymph nodes outside pelvis, bones, or viscera). The association between two categorical variables was evaluated by Chi-Square (χ2) test. Student’s t-test was used to compare continuous variables between the groups. A p-value of less than 0.05 was considered as statistical significance for -square (χ2) test and student’s t-test. Local control, survival, and late complication rates were calculated by the Kaplan Meier method, and differences between groups were compared by the log-rank test. A p-value of less than 0.05 was considered to indicate statistical significance. Statistical analysis was done using the Statistical Package for Social Sciences, version 20.
Results
Characteristics of the patients
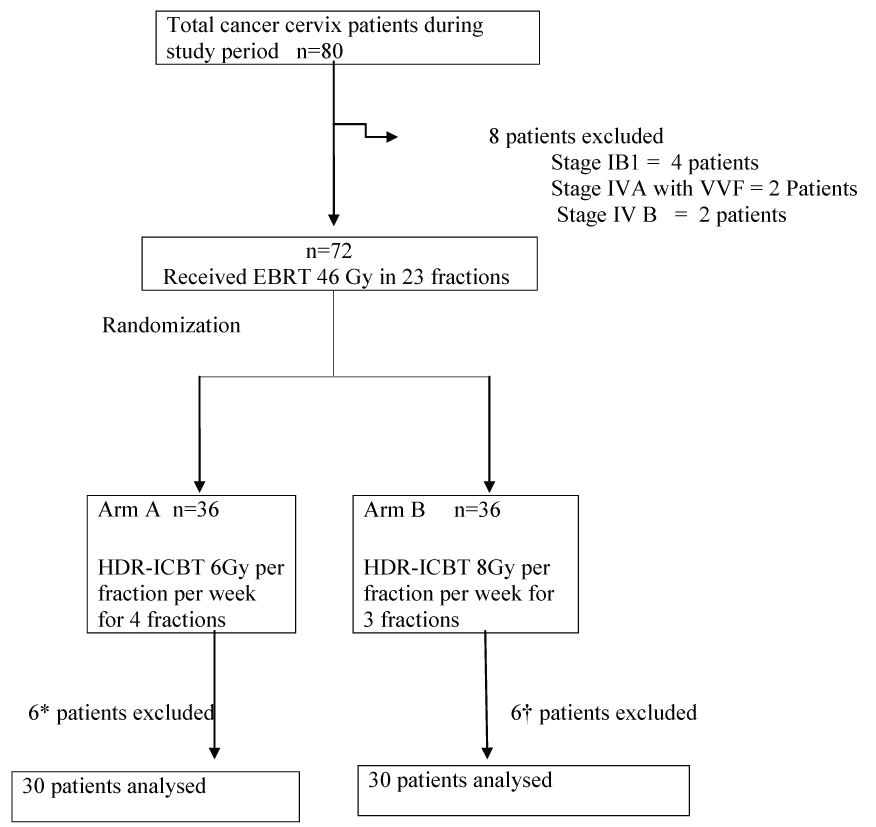
During the study period from March 2013 to August 2014, 80 patients with carcinoma cervix attended to radiotherapy department in our institute. Out of 80 patients, 8 patients are excluded (4 patients had Stage IBI, 2 patients had stage IV disease with Vesico-Vaginal Fistula (VVF), 2 patients had distant metastasis at presentation). Thus, 72 eligible patients were included in this study and they were randomized to either of the two arms by computer generated random number tables. Schema of section, randomization and allocation of patients into two arms were shown in [Table/Fig-2]. In arm A, five patients not received 4th fraction of HDR-ICRT and one patient was not available for follow up. In arm B, three patients defaulted after EBRT, three patients lost to follow up. The remaining total number of 60 patients of 30 in each arm was analyzed in this study. Prognostic factors were well balanced between the two treatment groups after randomization. The median age of the patients was 50 years all patients in both arms belongs to stage II and stage III. Histopathology of all cases was squamous cell carcinoma. The mean haemoglobin level was 10gm% in Arm A and 9 gm% in Arm B. The characteristics of patients in two arms were shown in [Table/Fig-3].
Schema of selection, randomization and allocation of patients into two arms.
n= number of patients.
* In arm A, 5 patients not received 4th fraction of HDR-ICRT and one patient are not available for follow up.
†In arm B, 3 patients defaulted after EBRT, 3 patients lost to follow up
Characteristics of patients.
| Patient characteristic | Arm A | Arm B |
|---|
| Age group | | |
| Median age, yr (range) | 50 years (range 37-78) | 51 years (38-75) |
| <50 | 13 | 8 |
| >50 | 17 | 22 |
| Size of the lesion | | |
| <4cm | 13(43.3%) | 12(40%) |
| >4cm | 17(56.6%) | 18(60%) |
| FIGO stage | | |
| II | 23 (76.6%) | 17 (56.6 %) |
| III | 7(23.3%) | 13 (43.3%) |
| Differentiation of SCC* |
| Well | 19 | 16 |
| Moderate | 7 | 9 |
| Poor | 1 | 3 |
| Unspecified | 3 | 2 |
| Chemo received | | |
| Yes | 20(66.6%) | 14(46.6%) |
| No | 10(33.3) | 16(53.3%) |
| Median OTT | 53 days (48-81) | 48 days(40-58) |
OTT-overall treatment time, *SCC-Squamous cell carcinoma
Treatment Parameters
All patients in both arms received pelvic EBRT dose of 46 Gy in 23 fractions at the rate of 2 Gy per fraction. Arm A recieved 6 Gy per fraction per week x 4 fractions and Arm B received 8 Gy per fraction per week x 3 fractions. Total 20 patients in arm A and 16 patients in arm B received concurrent weekly cisplatin chemotherapy. Total rectum BED3 was 110 Gy (97.4-137) in arm A and 111Gy (94.4 -142). Total bladder BED3 was 110Gy (92.8-143) in arm A and 125Gy (93.8-145) in arm B. The median overall treatement time was 53 days (48-81) in Arm A and 48 days (40- 58) in Arm B.
The median follow-up for the whole group at the time of analysis was 30 months (22 to 35). The median follow up for Arm A was 30 months (23 to 35) and for Arm B was 26 months (22 to 35).
Local control and disease-free survival
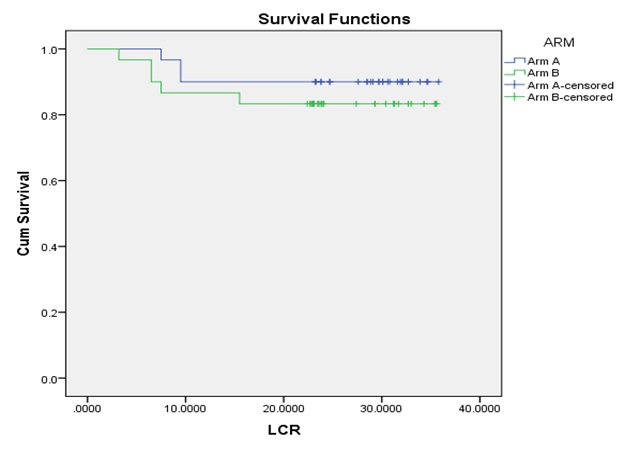
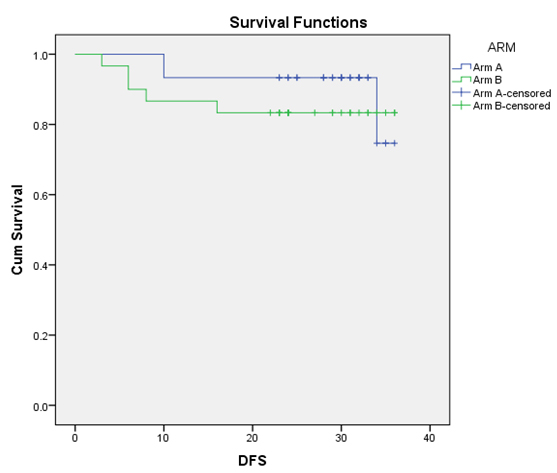
The local control rate for 30 months in Arm A was 90% and 83.3% in Arm B which was calculated by Kaplan Meier method [9] and it was shown in [Table/Fig-4]. The differences of local control between the two arms were compared by the log-rank test and it was not statistically significant (p= 0.21). The disease-free survival for 30 months in Arm A was 90% and 83.3% in Arm B which was calculated by Kaplan Meier method and it was shown in [Table/Fig-5]. The differences of disease-free survival between the two arms were compared by the log-rank test and it was not statistically significant (p= 0.39).
Local Control Rates (LCR) for 30 months in arm A and arm B.
LCR: 90% in Arm A and 83.3% in Arm B (p= 0.21).
LCR graphs of two arms plotted by Kaplan Meier method and difference in two arms computed by log-rank test
Disease Free Survival (DFS) for 30 months in arm A and arm B.
DFS for the 30 months - 90% in Arm A and 83.3% in Arm B (p= 0.39).
DFS graphs of two arms plotted by Kaplan Meier method and difference in two arms computed by log-rank test
Patterns of failure
Two patients (6.6%) in Arm A and three patients (10%) in Arm B developed loco regional recurrence. One patient (3.3%) in Arm A and three patients (10%) in Arm B developed distant failure. The characteristics of patients developing loco regional recurrence were shown [Table/Fig-6]. The characteristics of patients developing distant failure were shown [Table/Fig-7].
Characteristics of patients developing Loco regional recurrence in between two arms.
| S.N | Arm | Site of recurrence | Time of recurrence in months | Age | Stage | Hb prior to RT | Chemo received | OTTdays |
|---|
| 1 | A | cervix | 9.5 | 48 | IIB | 11 | No | 50 |
| 2 | A | cervix | 9.5 | 65 | IIB | 7 | No | 63 |
| 3 | B | cervix parametrium | 6.5 | 50 | IIIB | 8 | Yes (5cycles) | 36 |
| 4 | B | Cervix, vagina | 6.5 | 58 | IIIA | 8 | Yes (4cycles) | 70 |
| 5 | B | cervix, inguinal, nodal recurrence | 7 | 69 | IIIB | 6 | No | 58 |
Hb:haemoglobin, RT: Radiotherapy, OTT: overall treatment time
Characteristics of patients with distant failure in two arms.
| S. No. | Arm | Site of recurrence | Time of recurrence | Age | Stage | Hb prior to RT | Chaemo received | OTT days |
|---|
| 1 | Arm A | Para aortic nodes | 7.5 | 40 | IIIB | 7 | N | 47 |
| 2 | Arm B | Lungs, bones | 9.5 | 60 | IIB | 9 | Y | 45 |
| 3 | Arm B | supraclavicular | 11.5 | 69 | IIIB | 6 | N | 58 |
| 4 | Arm B | Para-aortic nodes lungs | 11.5 | 55 | IIB | 9 | N | 45 |
Hb:haemoglobin, RT: Radiotherapy, OTT: overall treatment time
Acute toxicity
During course of radiation, acute toxicities were evaluated according to the National Cancer Institute; Common Terminology Criteria of Adverse Event (CTCAE) version 4. Acute toxicities related to hematologic profiles, gastro intestinal and genitourinary toxicity were evaluated. No patient showed grade 3 or 4 toxicity in terms of the haematologic, gastrointestinal, or genitourinary systems. All acute toxicities were relieved spontaneously or controlled with minor medications.
Late toxicity
Late toxicity was graded according to the Radiation Therapy Oncology Group Criteria [8]. The four patients (13%) patients in Arm A and eight patients (26%) in Arm B developed late rectal toxicity.(p=0.5). Three patients (10%) patients in Arm A and five patients (16%) in Arm B developed late bladder toxicity (p=0.43)
Late bladder toxicity: Two patients (6.6%) developed Grade-I, only one patient (3.3%) developed grade II toxicity in arm A. Three patients (10%) developed Grade-I toxicity and only two patient (6.6%) developed grade II toxicity in arm B. Mean time to develop bladder toxicities: 12 months (11-12 months) in Arm A and 14 months (13-18 months) in Arm B (p=0.5). The median total bladder BED received by patients in Arm A from EBRT and three brachytherapy sessions was 110Gy (92.8-143), and in Arm B, it was 125Gy (93.8-145) from EBRT and all four brachytherapy sessions. Range of BED3 in patients developing bladder toxicity was 125 -142Gy3. All patients had BED 3 more than 125 Gy3.
Late rectal toxicity: Three (10%) patients developed Grade-I toxicity and only one patient (3.3%) developed grade II toxicity in arm A. Four (13.3%) patients developed Grade-1 toxicity and three patients (10%) had grade –II, toxicity and only one patient (3.3%) developed grade III toxicity. The median total rectal BED received by patients in Arm A from EBRT and three brachytherapy sessions was 110 Gy3(97.4-137) and in Arm B, it was 108.51 Gy3 from EBRT and all four brachytherapy sessions was 111Gy3 (94.4 -142). Range of BED3 in patients developing rectal toxicity 92-142Gy3. Only two patient in arm A and two patients in Arm B more than BED3 125Gy3. charecterstics of patients devloping bladder and rectal toxicities were shown in [Table/Fig-8].
Late bladder and rectal toxicities in between two arms.
| Latetoxicity | Arm An (%) | BED3 | Mediantime to developtoxicity | Arm Bn(%) | BED3 | Mediantime todeveloptoxicity |
|---|
| Bladdertoxicity | Grade 1 | 2(6.6%) | 142130 | 12 m(11-16m) | 3(10%) | 125133133 | 15(13-15m) |
| Grade 2 | 1(3.3%) | 126 | 2(6.6%) | 142141 |
| Rectaltoxicity | Grade 1 | 3(10%) | 126127121 | 13.5 m(11-16m) | 4(13.3%) | 126127121112 | 16m(11-13m) |
| Grade 2 | 1(3.3%) | 132 | 3(10%) | 109114101 |
| Grade 3 | - | - | 1(3.3%) | 113 |
n = number of patients
Discussion
Combined radiotherapy approach in the form of external beam radiotherapy and intracavitary brachytherapy has been accepted as the standard of radical treatment in uterine cervical cancer worldwide. The incorporation of brachytherapy enhances the curative potential by dose escalation after EBRT and delivers high dose directly to tumour while sparing surrounding normal structure. Although, HDR brachytherapy has practical advantage of shorter treatment time, low radiation hazard to staff, dose optimization, but there is a large concern about late toxicity due to large dose per fraction. A study by Orton et al., who done an analysis on the data obtained from a survey of 56 institutions treating a total of over 17,000 cervix cancer patients with HDR-ICBT, found that patient morbidity rates were significantly lower for Point A doses/fraction < 7 Gy compared with > 7 Gy for both severe injuries (1.28% vs. 3.44%) and moderate plus severe (7.58% vs. 10.51%) [10]. They demonstrated that fractionation of the HDR treatments significantly influenced toxicity. But, this study by Ortan et al., is a retrospective analysis, and only physical doses were taken into consideration, not BED values, and toxicity scoring was done by morbidity criteria [10]. In further analysis, Orton et al., stated that 4 to 9 Gy can be acceptable fractionation range, but proper packing/retraction technique in ICBT and midline blocking in EBRT technique are important to decrease dose to central normal structures there by late complications [11]. Petereit et al., reviewed 24 articles on HDR brachytherapy for cervical cancer using different regimens tried to correlate BED10 and BED3 to pelvic control and complications, respectively [12]. But, no dose-response relationship for tumour control (median BED10- 96 Gy3) or late tissue complications was noted. They observed that the technique and experience of individual centres might have played a more important role than attempts to optimize fractionation. The ABS recommends individual fraction size of less than 7.5 Gy per fraction using 4 to 8 fractions. However, ABS also includes a caution that these guidelines are no substitute for clinical experience and need to be tested in clinical setting. In the literature, various [13-18] studies compared different fractionation schedules in HDR-ICRT, but the doses of EBRT to the whole pelvis differ widely in their studies. So, simple comparison of fraction size and total physical dose, may lead to incorrect interpretation of results. Since, the concept of BED was accepted in the clinical field, some have reported upon the results of various combinations of EBRT and ICBT fractionations in terms of BED10 or BED3. Ferrigno et al., noticed that the 5-yr late bladder complication rate was higher among patients treated with BED of larger than 125 Gy3 at bladder reference point, although the difference was not statistically significant (17% vs. 9%, p=0.27) [19]. Toita et al., recommended that the rectal BED3 should be kept below 100-120 Gy [20]. Clark et al., found a dose response relationship and a threshold for late rectal complications above 125 Gy3 at rectal reference point, in a study involving concurrent chemo radiotherapy [21]. Ogino et al., not showed any grade IV rectal complications even with dose to rectum was equivalent or less than 147Gy [22]. In our study, the range of rectal BED3 to point A, in patients developed rectal toxicity was 96 to 145Gy Gy. The range of bladder BED3 to point A for patients developed bladder toxicity was 125-145Gy in our study. Total 12 patients in our study developed rectal toxicity, but out of 12 patients 9 patients having BED3 less than 120 Gy. No correlation was found between BED3 to rectal and bladder complications.
Patel et al., done a prospective randomized study in 104 cervical cancer patients treated with external beam and HDR [23], either 9 Gy for two fractions(Arm A) or 6.8 Gy for three fractions(Arm B) each fraction 1 week apart. The 3-year actuarial risk of developing any grade 3 or worse late toxicity was 7.47% with 9 Gy and 3.57% with 6 Gy (p = 0.3).They further states that despite using high dose per fractions (9 Gy per fraction in arm A), incidence of major late toxicity were low, because of doing application under general anaesthesia and effective vaginal packing. They concluded that fewer fractions are more economical by reducing number of hospital admissions.
Limitation
Small sample size and short follow-up.
Conclusion
In our study, two fractionation schedules showed comparable treatment outcomes and toxicity. So, both schedules seem to be safe and effective. With supporting literature, we can say, fraction size may not influence the local control and late toxicity. Taking into account of increased hospital burden of locally advanced cancer cervix patients in Indian context, high dose rate intracavitary brachytherapy schedule of 8 Gy per fraction per week x 3 fractions is the preferable option over 6 Gy per fraction per week x 4 fractions with regard to comparable loco-regional control, acute and late toxicity, disease free survival and better patients compliance to lesser fractionation schedule.
FIGO- International Federation of Gynaecology and Obstetrics