Rotenone, a pesticide is a well known mitochondrial complex I inhibitor [1]. Being highly lipophilic, this toxin easily crosses the blood brain barrier and does not need any transporter to enter the dopaminergic neuron, unlike other neurotoxins such as 6-hydroxydopamine, MPTP and paraquat [2,3]. Rotenone induced neurotoxin model of PD is the most recently studied among other neuro toxic agents [4]. Bilateral lesion by rotenone in medial forebrain bundle caused the selective degeneration of nigrostriatal dopaminergic neurons and appears to produce PD symptoms in rats [5]. Intrastriatal administration of rotenone in rats can mimic the motor dysfunction and neuropathological features of human PD, while being less toxic than systemic administration [6]. Since bilaterally lesioned animals will be requiring more postoperative care because of the feeding difficulty and may increase mortality rate, hemiparkinsonian animal model are more suitable for evaluating behavioural parameters and can be used in the development of neuroprotective and neurotrophic treatment strategies [7]. The unilateral ‘partial’ lesion model closely mimic the mid-stage PD in which striatum still possess dopaminergic innervations due to 80% loss of nigral neurons. The ‘complete’ unilateral lesion model mimics only the end-stage Parkinsonism due to loss of more than 95% of nigral neurons [8]. Hence, we used rotenone which is stereotactically injected into the striatum at two different sites to induce unilateral partially lesioned rat model of PD. Only a low dose of 2.5 μg of rotenone is required to achieve 80% loss of dopamine content in striatum [9]. But low doses take at least 90 days for a moderate depletion. The present study was designed to assess rotenone toxicity by behavioural measures and by histology at a moderate dosage of 25 μg/site. Our aim was to find whether rotenone at this dose can produce consistent behavioural measures and a consistent lesion in striatum. Rotenone lesioned hemiparkinsonian animals are compared to sham treated and L-Dopa treated with respect to their motor response and histopathological data.
Materials and Methods
Animal Experimental Study: The project was approved by IAEC (Institutional Animal Ethical committee) University of Madras, Chennai, India and Committee for Purpose of Control and Supervision of Experiments on Animals (CPCSEA), and was conducted in accordance with the standard procedures of the IAEC. The project approval number is IAEC No.01/017/04. It was conducted in Department of Anatomy, University of Madras, Chennai, India from August to December 2009. Healthy adult male Wistar albino rats weighing about 200 to 230 grams of body weight were used in this study. Rats were housed in pairs and maintained under standard atmospheric condition of 12 hours light/12 hours dark cycle at 21°C to 26°C and 30 to 60 percent humidity. Animals were fed with standard rat pellet diet (Hindustan lever limited, Mumbai, India) and water ad libitum.
Drugs and Chemicals: Rotenone, Dimethyl sulphoxide, Polyethylene glycol, Levodopa methyl ester, Benserazide hydrochloride, Desipramine HCl, Haematoxylin and Eosin (H&E), Cresyl Fast Violet (CFV), Silver nitrate were procured from Sigma-Aldrich, Germany, Sodium pentobarbital (Abbott Laboratories, Germany).
Experimental Design: Rats were divided into three groups. Group I sham control received 1μl of equal volume of DMSO and PEG in the ratio of 1:1 at two different infusion sites of striatum. Group II received 25 μg of rotenone in 1 μl of DMSO: PEG in the ratio of 1:1 for each site in striatum and Group III rotenone lesioned animals treated with L-Dopa.
Animals of I and II Group were sacrificed on 21st and 30th day. On day 21, Group III lesioned animals were treated with L-Dopa for a period of 10 days. They were sacrificed after three hours of last dose of administration of L-Dopa on 30th day. Animals of three groups were used for histological study after the behavioural observation.
Surgical Procedure: The animals were anaesthetized by intra-peritoneal administration of sodium pentobarbital at the dose of 40 mg/kg body weight [10]. Then the rats were immobilized in a stereotaxic frame (Stoelting, USA) in the flat skull position with incisor bar set at -3.3 mm. Two holes were drilled on the same side over the dorsolateral part and dorsomedial part of the striatum on the skull surface. The dorsolateral part of the striatum was reached by following co-ordinates of Antero posterior (AP)=0.2 mm, Mediolateral (ML) =3.2 mm, Dorsoventral (DV)=4.5 mm from the Bregma and the dorsomedial part of striatum was reached by the co-ordinates of AP=1.1 mm, ML=2.4 mm and DV=3.5 mm using the stereotaxic atlas of Paxinos and Watson [11]. The injection volume was 1 μl for each site and was made manually with the help of a 10 microlitre Hamilton syringe (P/N: 80300/00 Hamilton Bonaduz AG, CH-7402, Switerzerland), through the burr holes made on the skull surface in Groups I, II and III. The infusion rate was 0.2 μl/min and the needle was kept in place for an additional 5 minutes to allow diffusion. The hole was closed using bone wax (W810, Ethicon, Johnson and Johnson, India Ltd.,) immediately after the withdrawal of the needle from the striatum. To prevent the uptake of rotenone by non-adrenergic terminals, desipramine HCl (25 mg/kg.b.wt) was administered intraperitoneally 30 minutes before the rotenone injection. The incision was sutured and proper postoperative care was taken until the animals recovered completely.
Experimental Procedures: On the 21st day after lesion, a dose of (10 mg/kg b.wt) L-DOPA treatment (i.p.) combined with peripheral amino acid decarboxylase inhibitor benserazide hydrochloride were daily administered for a period of 10 days in the lesioned group. Benserazide hydrochloride (one-quarter dose of L-DOPA) was administered (i.p.) 30 min before the treatment with L-DOPA [5].
Behavioural Testing: Animals were trained for seven days before lesion and the behavioural tests were started seven days after lesion at intervals of seven days over a 21 day period for the animals of the Group I, II and III. On the 21st day treatment was started for the Group III. The rotarod and open-field behavioural tests were done and compared with the lesioned and sham control groups on 30th day. The experiments were performed between 9.00 am and 14.00 pm at standard conditions. Behavioural activities were recorded by the video graph for all the groups.
Spontaneous Circling Behaviour: The animals were kept in a transparent cage 24 hours following surgery and recovery from anaesthesia the spontaneous rotations (360° in a short axis) in the cage were observed and counted for one hour [7].
Rotarod Test: The animals were placed on the Rota rod cylinder and the time the animal remained in the Rota rod was measured at the constant speed of 15 rpm for maximum three minutes. Three trials were conducted and the mean duration on the rod was recorded [12–14].
Open Field Test: An open square wooden box measuring 100 x 100 x 40 cm was illuminated with the 100 watts bulb kept suspended at 1.5 meter distance from the centre of the box. The floor of the box was divided into 25 equal squares. It consists of 16 squares in the periphery and 9 squares in the centre. Each animal was placed in the centre square of the open field and following parameters were measured for 5 minutes [15].
locomotion in peripheral squares (number of entries);
locomotion in centre squares (number of entries);
rearing (time in seconds);
immobilisation (time in seconds);
grooming (time in seconds).
Histology: The animals were transcardially perfused with Phosphate Buffer Saline (PBS) followed by 4% Para formaldehyde in PBS. The brains were removed immediately and stored in 4% Para formaldehyde in PBS for 24 hours and were processed for paraffin embedding. Sections were cut at 10 μm thickness and stained with the CFV, H&E stain [16] and Silver Nitrate [17]. Histological details were observed and micro photographed.
Statistical Analysis
All data were expressed as mean±standard error of the mean (Mean±SE). Statistical analysis was carried out by One-way Analysis of Variance (ANOVA) using SPSS software, version-7.0 i.e., analysis of variance. The analysis of variance is followed by Tukey’s test and values of p< 0.05 were considered statistically significant.
Results
Behavioural Observation of Rotenone lesioned animals Spontaneous circling behaviour:
After recovery from anaesthesia, all the rats infused with rotenone into striatum exhibited spontaneous ipsilateral rotational behaviour to the side of infusion. No contralateral spontaneous rotation was observed. The spontaneous rotations were 100±20 for one hour duration. The intensity of this behaviour persisted over 45 minutes and there after slowly declined and vanished by one hour.
Rotarod Test
The duration of endurance of rotarod in Group II animals were significantly lesser than the Group I (p< 0.05). On the day 30, Group III animals do not show any significant difference when compared to Group I. When compared to the rotenone lesioned group, treated group animals showed significantly enhanced endurance time on the rotarod [Table/Fig-1].
Rotarod status after rotenone lesion from 7th to 30th day and on 30th day after 10 days of L-DOPA treatment. Values were measured in sec. Each value represents mean±SE six rats: a) comparison between rotenone and sham; b) comparison between rotenone and L-DOPA; c) comparison between treated and sham. Statistical comparisons were made using One-way ANOVA with Tukey’s test. *p<0.05 is significant.
| Groups | 7th day | 21st day | 30th day |
|---|
| Sham | 50.19±0.38 | 41.89±0.31 | 36.75±0.24 |
| Rotenone | 10.34±0.12a* | 10.27±0.15a* | 10.92±0.12a* |
| Rotenone+L-DOPA | 10.46±0.13a* b | 10.38±0.12a* b | 29.75±0.27a b* c |
Open Field Test (OFT)
The L-DOPA treated group did not show any significant difference in all sensory motor behaviours in open field test when compared to sham control on 30th day. When lesioned animals were placed in open field, peripheral square entries were decreased from 7th to 21st day, 21st to 30th day in Group II, when compared with the Group I indicated their decreased motor activity [Table/Fig-2]. In treated animal, the number of entries into peripheral squares was significantly increased (p<0.05) on 30th day compared to lesioned group.
Peripheral square status after rotenone lesion from 7th to 30th day and on 30th day after 10 days of L-DOPA treatment. Values were measured square entry in numbers/5min. Each value represents mean±SE six rats: a) comparison between rotenone and sham; b) comparison between rotenone and L-DOPA; c) comparison between treated and sham. Statistical comparisons were made using One-way ANOVA with Tukey’s test.*p<0.05 is significant.
| Groups | 7th day | 21st day | 30th day |
|---|
| Sham | 50.19±0.38 | 41.89±0.31 | 36.75±0.24 |
| Rotenone | 10.34±0.12a* | 10.27±0.15a* | 10.92±0.12a* |
| Rotenone+L-DOPA | 10.46±0.13a* b | 10.38±0.12a* b | 29.75±0.27a b* c |
The central square entries for lesioned animals showed decreased entries compared to control animals. The treated group showed significant difference, when compared to lesioned group on 30th day [Table/Fig-3].
Central square status after rotenone lesion from 7th to 30th day and on 30th day after 10 days of L-DOPA treatment. Values were measured square entry in numbers/5min. Each value represents mean±SE six rats. a) comparison between rotenone and sham; b) comparison between rotenone and L-DOPA; c) comparison between treated and sham. Statistical comparisons were made using One-way ANOVA with Tukey’s test.*p <0.05 is significant.
| Groups | 7th day | 21st day | 30th day |
|---|
| Sham | 3.19±0.10 | 4.62±0.11 | 4.15±0.13 |
| Rotenone | 2.86±0.14a* | 3.14±0.17a* | 2.13±0.12a* |
| Rotenone+L-DOPA | 2.86±0.12a* b | 3.09±0.15a* b | 3.98±0.18a b* c |
The number of rearing in lesioned animals was significantly reduced on 21st day (p<0.05) when compared to sham group and in L-Dopa treated group showed significant increase in number of rearing on 30th day [Table/Fig-4].
Rearing status after rotenone lesion from 7th to 30th day and on 30th day after 10 days of L-DOPA treatment. Values were measured rearing in numbers/5min. Each value represents mean±SE six rats. a) comparison between rotenone and sham; b) comparison between rotenone and L-DOPA; c) comparison between treated and sham. Statistical comparisons were made using One-way ANOVA with Tukey’s test.*p<0.05 is significant.
| Groups | 7th day | 21st day | 30th day |
|---|
| Sham | 14.33±1.15 | 12.50±0.88 | 12.73±0.75 |
| Rotenone | 07.73±0.50a* | 07.50±0.57a* | 08.15±0.32a* |
| Rotenone+L-DOPA | 07.59±0.75a* b | 07.57±0.55a* b | 11.67±0.45a b* c |
The sham group showed more movements in open field test and hypokinesia was very less. The rotenone lesioned animals showed significant increase in its immobilization on 7th day and higher significant increase in its immobilisation on 21st day (p<0.05) when compared to the control group. However, on 30th day the treated group of animals showed significant improvement in its locomotion, when compared to lesioned group [Table/Fig-5].
Immobilisation status after rotenone lesion from 7th to 30th day and on 30th day after 10 days of L-DOPA treatment. Values were measured in sec/5min. Each value represents mean±SE six rats. a) comparison between rotenone and sham; b) comparison between rotenone and L-DOPA; c) comparison between treated and sham. Statistical comparisons were made using One-way ANOVA with Tukey’s test.*p <0.05 is significant.
| Groups | 7th day | 21st day | 30th day |
|---|
| Sham | 155.53±5.35 | 150.50±4.42 | 153.33±2.33 |
| Rotenone | 220.84±12.23a* | 207.00±14.80a* | 207.14±18.84a* |
| Rotenone+L-DOPA | 220.95±12.21a* b | 207.09±14.84a* b | 189.32±7.57a b* c |
In grooming, the lesioned animals showed significant reduction in grooming number during all test periods (p<0.05), when compared to the sham control animals. In treated group, the number of grooming was increased significantly on 30th day when compared to lesioned group day [Table/Fig-6].
Grooming status after rotenone lesion from 7th to 30th day and on 30th day after 10 days of L-DOPA treatment. Values were measured grooming in numbers/ 5min. Each value represents mean±SE six rats. a) comparison between rotenone and sham; b) comparison between rotenone and L-DOPA; c) comparison between treated and sham. Statistical comparisons were made using One-way ANOVA with Tukey’s test.*p<0.05 is significant.
| Groups | 7th day | 21st day | 30th day |
|---|
| Sham | 4.19±0.12 | 5.70±0.14 | 6.50±0.16 |
| Rotenone | 2.81±0.09a* | 2.77.±0.11a* | 3.27±0.14a* |
| Rotenone+L-DOPA | 2.80±0.09a* b | 2.76±0.12a* b | 5.13±0.14a b* c |
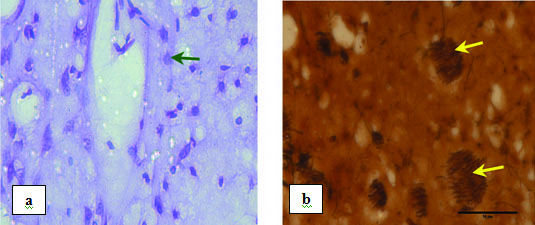
Sham control (Vehicle injected) corpus striatum
The striatum of sham group animals did not show any neuronal degeneration around the vehicle injected site [Table/Fig-7] but showed only a small area of loss of dopaminergic fibers along the needle track. There were no changes in neuronal morphology on both the sides of the striatum. This observation indicates that there was no considerable impact by the vehicle injection. The contralateral side of sham control striatum served as a control.
Photomicrograph showing sham control striatum magnified under 40X: a) Stained with CFV (Arrow indicates normal neuron); b) Stained with silver nitrate. Yellow arrows indicate normal bundle of nerve fibres.
Rotenone lesioned corpus striatum
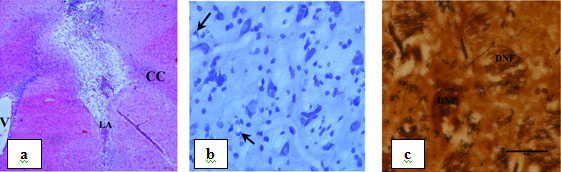
The extent and location of the lesions after intrastriatal injection of rotenone at two sites in coronal sections of the forebrain were confirmed by H&E, Silver and Nissl stain. The intrastriatal injections of rotenone produced consistent and discrete lesions of the striatum. The degeneration of dopaminergic fibres, gliosis and progressive loss of the striatal neurons were observed in the ipsilateral striatum around the injected site on 21st day [Table/Fig-8]. The cell density of the neuron was reduced significantly on 21st day when compared with the sham control group.
Photomicrograph showing striatum 20 days after rotenone lesion: a) Stained with H&E magnified under 4X (CC – Corpus Callosum, LA – Lesioned Area. V-Ventricle); b) Stained with CFV magnified under 40X. Arrows indicating degenerating neuron; c) Stained with silver nitrate magnified under 40X. DNF – Degenerating nerve fibre.
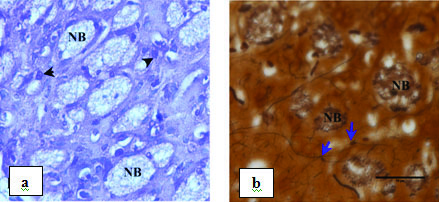
In L-DOPA treated group, there was a relative inhibition of neuronal cell death. There was increase in bundle of nerve fibres making new connections and reduction in number of activated glial cell when compared to the lesioned group on 30th day [Table/Fig-9].
Photomicrograph showing striatum on 30th day of L-Dopa treatment after 20 days of rotenone lesion magnified under 40X: a) Stained with CFV. NB – regenerated nerve bundle. Arrow heads indicating normal neuron; b) stained with silver nitrate. NB- regenerated nerve bundle. Arrows indicating nerve fibre making new connection.
Discussion
Effect of rotenone exposure on behaviour
In the present study, the striking feature is that the unilateral infusion of rotenone at a dose of 25 μg/1 μl into each site of the striatum of rats resulted in no mortality, whereas mortality rate is higher in bilateral lesioned model treated systemically [18].
The spontaneous ipsilateral circling behaviour in rats infused with rotenone soon after recovery from anaesthesia was an interesting observation. The spontaneous ipsilateral circling behaviour observed in the present study is probably due to release of DA into the ipsilateral striatum after rotenone infusion. Since rotenone is a slow acting toxin, injection at a low dose requires longer duration to achieve moderate DA depletion [7,19]. Hence, this spontaneous ipsilateral circling behaviour in the drug free state has not been reported so far. On the contrary, spontaneous contralateral circling behaviour following unilateral intranigral infusion of the rotenone has been reported [20]. Unilaterally lesioned models exhibit behavioural deficits due to neurotransmitter imbalance between the two sides and will lead to rotational behaviour. Similar to the previous studies [14,21], we also observed that the duration of endurance period of Group II animals on the rotarod was significantly shorter than the Group I may be due to the loss of dopaminergic neurons. In open field, the decrease in entries and distance travelled by lesioned rats indicated decreased motor activity which supports the previous work [22]. The reduced values of grooming and rearing are observed in the present work might be due to the depletion of DA content and causes metabolite deficiency in the nigrostriatal dopaminergic neurons. On the contrary, the study showed that there were no significant differences in grooming6-hydroxydopamine lesion in the striatum, when compared to the control [15]. Among all the behavioural measures studied, locomotor activity, movement initiation and postural stability were found to be inconsistent except rearing after systemic administration of rotenone in rats [23]. All the neurobehavioural measures including head dips and forelimb steps in rotenone lesioned animals for every 10 days over a period of 120 days after lesion were found to be consistent. The signs and symptoms pertaining to Parkinsonism appeared on 7th day, highest on 21st day, highly significant on 60th day and least significant on 120th day when compared to sham control group (unpublished data).
In our findings, all rotenone lesioned animals showed increase in immobilization, decrease in locomotor activity, rearing, grooming and balance were reversed after administration of Levodopa treatment may be due to the activation of dopaminergic neurons, sprouting or regrowing of nerve terminals through the Levodopa. It was reported that except rearing all the other behavioural changes like decrease in locomotor activity, head dips and inactive sitting were reversed after chronic administration of Levodopa in bilateral rotenone lesioned medial forebrain bundle of rats [5].
Effect of Rotenone on Striatum
Our histological study of striatum showed that the Dimethyl sulphoxide /Polyethylene glycol-300 which is commonly used as solvents in biological studies and as vehicles for drug therapy did not affect the striatal neurons which supports the previous reports [24]; but very few loss of nerve terminals around the injected site. The intrastriatal injection of DMSO did not show significant changes in DA and their metabolites with respect to control striatum and the general appearance of the tissue was normal even around the injection site [25]. Localized striatal lesions may be a more consistent method of producing partial lesions, because in striatum there is a high density of dopaminergic fibers that allows the diffusion of the toxin to be more easily controlled [26].
Hypertrophied neurons, degenerating neurons, damage to the striatal nerve terminals and gliosis are observed in rotenone lesioned striatum. We also found the reduction in the number of neurons and the density of nerve fibres are higher on 60th day than on 120th day and the highest on 21st day in lesioned animals when compared to sham control (unpublished data). It suggest that the rotenone is a mitochondrial complex I inhibitor produced selective damage in the striatum. The continuous intravenous infusion of rotenone causes highly selective dopaminergic lesions, but the striatal nerve terminals were affected earlier and more severely by rotenone than substantia nigra which occured later in a retrograde manner [18]. Even with an 80% loss of dopamine input in the nigra, the residual dopaminergic terminals in the striatum can maintain normal extracellular dopamine levels may be due to increase in dopamine synthesis and release, tyrosine hydroxylase production and sprouting of residual terminals [27].
In levodopa treated Group, there was a relative inhibition of neuronal cell death, as well as striatal neurons exhibited a pronounced capacity for sprouting nerve terminals, increase in bundle of nerve fibres and reduction in number of activated glial cell. The present data indicate that administration of L-DOPA after unilateral lesion is followed by an improvement of behavioural and histological parameters which define the nigro-striatal dopaminergic pathways.
Limitation
The limitation of this study is that even though the open field and rotarod test have given better behavioural results; the simultaneous or independent use of right or left forelimb: (i) for vertical exploration; (ii) for landing after a rear; and (iii) no. of steps used could not be assessed in aforementioned tests for a hemiparkinsonian animal model. If assessed by Schallert and Tillerson method, it might have made our results more robust without impairing the interpretation of the data.
Conclusion
Hemiparkinsonian partial animal model produced through unilateral intrastriatal rotenone administration in two sites showed no mortality. It confirms that the rotenone, a neurotoxin is less toxic at this single dose and produced a suitable PD model for evaluating behavioural parameters even for long term period. It produced more consistent striatal lesion. Since this lesion developed better behavioural manifestations and are more near to human idiopathic PD, are suitable for studying pathophysiology of the disease. We found that this model is also more susceptible to L-DOPA. We conclude that a single dose of intrastriatal administration of rotenone induced hemiparkinsonian partial animal model without any mortality might be used as one of the best suitable model for development of new therapies in long course of treatment to halt or reverse neurodegenerative process.
Mesh Terms
6-OHDA - 6-hydroxydopamine, MPTP - 1-methyl4-phenyl-1,2,3,6-tetrahydropyridine, IAEC- Instituional Animal Ethical Committee, PD – Parkinson’s Disease, H&E – Haematoxylin and Eosin, CFV – Cresyl Fast Violet, PBS- Phosphate Buffered Saline, S.E – Standard Error, DA - Dopamine, DMSO - Dimethyl sulphoxide, PEG – Polyethylene glycol, L-Dopa - Levodopa
[1]. Xiong N, Long X, Xiong J, Jia M, Chen C, Huang J, Mitochondrial complex I inhibitor rotenone - induced toxicity and its potential mechanisms in Parkinson’s disease modelsCrit Rev Toxicol [Internet] 2012 42:613-32. [Google Scholar]
[2]. Gutman M, Singer TP, Beinert H, Caside JE, Reaction sites of rotenone, piercidin A, and amytal in relation to the nonheme iron components of NADH dehyrogenaseProceedings of the National Academy of sciences USA 1970 65(3):763-70. [Google Scholar]
[3]. Tieu K, A guide to neurotoxic animal models of Parkinson’s diseaseCold spring Harb Perspect Med 1 2011 1:a009316 [Google Scholar]
[4]. Subaraja M, Vanisree AJ, Rotenone causing dysfunctional mitochondrial and lysosomes in cerebral ganglions of Lumbricus terrestris degenerate giant fibre and neuromuscular junctionChemosphere [Internet] 2016 152:468-80. [Google Scholar]
[5]. Alam M, Mayerhofer A, Schmidt WJ, The neurobehavioural changes induced by bilateral rotenone lesion in medial forebrain bundle of rats are reversed by L-DopaBeh brain Res 2004 151(1):117-24. [Google Scholar]
[6]. P Walsh S, Paucard A, Rea K, Dowd E, Characterisation of a novel model of Parkinson’s disease by intrastriatal infusion of the pesticide rotenoneNeurosci 2011 181(2):234-42. [Google Scholar]
[7]. Saravanan KS, Sindhu KM, Mohanakumar KP, Acute intranigral infusion of rotenone in rats causes progressive biochemical lesions in the striatum similar to Parkinson’s diseaseBrain Res [Internet] 2005 1049(2):147-55. [Google Scholar]
[8]. JT Fiandaea MS, Kordower JH, Notter MFD, Gash DM, Striatal adrenal medulla/sural nerve cografts in hemiparkinsonian monkeysProg Brain Res 1990 82:573-80. [Google Scholar]
[9]. RE Nicklas WJ, Vyas I, Duvoisin RC, Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: implications for the mechanism of 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine toxicityNeurosci Lett [Internet] 1985 62(3):389-94. [Google Scholar]
[10]. Antkiewicz-Michaluk L, Wardas J, Michaluk J, Romanska I, Bojarski A, Vetulani J, Protective effect of 1-Methyl -1,2,3,4-Tetrahydroisoquinoline against dopaminergic neurodegeneration in the extrapyramidal structures produced by intracerbral injection of rotenoneInt J Neuropsychopharmacol 2004 7(2):155-63. [Google Scholar]
[11]. Paxinos G, Watson C, The rat brain in stereotaxic coordinates 1996 3rd edLondon NW 17DX, UKAcademic Press Ltd [Google Scholar]
[12]. Rozas GL, Garcia JL, Drug-free evaluation of rat models of parkinsonism and nigral graft using new automated rotarod testBrain Res [Internet] 1997 749(2):188-99. [Google Scholar]
[13]. Rozas G, Guerra MJ, Labandeira-Garcia JL, An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of ParkinsonismBrain Res Prot [Internet] 1997 2(1):75-84. [Google Scholar]
[14]. Rozas G, Martinn EL, Gierra MJ, Garcia JL, The overall rod performance test in the MPTP-treated mouse model of ParkinsonismJ Neurosci Methd 1998 83(2):165-75. [Google Scholar]
[15]. Fornaguera J, Schwarting RK, Time course of deficits in open field behaviours after unilateral neostriatal 6-hydroxydopamine lesionsNeurotoxi Res 2002 4(1):41-49. [Google Scholar]
[16]. Bancroft JD, Gamble M, Theory and practice of histological techniques 2002 5th edEdinburgh London Newyork Oxford PhiladephiaHarcourt Publishers Ltd [Google Scholar]
[17]. Culling CFA, Handbook of histopathological and histochemical techniques 1972 3rd edLondonButterworths [Google Scholar]
[18]. R Sherer TB, Mackenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT, Chronic systemic pesticide exposure reproduces features of Parkinson’s diseaseNat Neurosci 2000 3(12):1301-06. [Google Scholar]
[19]. Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, Sulforaphane protects against rotenone induced neurotoxicity in vivo: Involvement of mToR, Nrf2 and autophagy pathwaysSci Rep [Internet] 2016 6:32206 [Google Scholar]
[20]. Sindhu KM, Saravanan KS, Mohanakumar KP, Behavioural differences in a rotenone-induced hemiparkinsonian rat model developed following intranigral or median forebrain bundle infusionBrain Res [Internet] 2005 1051(1):25-34. [Google Scholar]
[21]. Jia F, Song N, Zhao C, Xie J, Jiang H, Unexpected improvements of spatial learning and memory abilities in chronic rotenone intoxicated micePLoS One [Internet] 2014 9(3):e91641 [Google Scholar]
[22]. Tamas A, Lubics A, Szalontay L, Lengvari I, Reglodi D, Age and gender differences in behavioural and morphological outcome after 6-OHDA induced lesion of the substantia nigra in ratsBehav Brain Res [Internet] 2005 158(2):221-29. [Google Scholar]
[23]. Fleming SM, Zhu C, Fernagut PO, Mehta A, Di Carlo CD, Seaman RL, Behavioural and immunohistochemical effects of chronic intravenous subcutaneous infusions of varying doses of rotenoneExp Neurol [Internet] 2004 187(2):418-29. [Google Scholar]
[24]. Garcia-Garcia Ponce S, Brown R, Cussen V, Krueger JM, Sleep disturbances in the rotenone animal model of Parkinson diseaseBrain Res 2005 1042(2):160-68. [Google Scholar]
[25]. Wu RM, Mohanakumar KP, Murphy DL, Chiueh CC, Antioxidant mechanism and protection of nigral neuron against MPP+toxicity by deprenyl (selegiline)Ann N Y Acad Sci 1994 (3):214-21. [Google Scholar]
[26]. Chang JW, Wachtel SR, Young D, Kang UJ, Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: Studies on medial forebrain bundle and striatal lesionsNeurosci 1999 88(3):617-28. [Google Scholar]
[27]. DI Stanic D, Parish CL, Tomas D, Dickson K, Horne MK, Axonal sprouting following lesions of the rat substantia nigraNeurosci 2000 97(1):99-112. [Google Scholar]