Preeclampsia is a pregnancy complication characterized by high blood pressure and proteinuria, usually occurs in the late second to third trimester. The incidence of preeclampsia estimated between 1.8%-16.7% of all pregnancies, which varies between countries [1]. Preeclampsia is a serious medical condition with multiple impacts to the maternal and foetal wellbeing. The Confidential Enquiries into Maternal Death persistently thus, causing a higher perinatal show substandard care in a significant percentage of the death morbidity and mortality rate [2]. In Indonesia, preeclampsia incidence is around 128.273 cases/year or about 5.3% [3]. A recent study of maternal death from 2003-2009, showed 343,000 maternal deaths due to hypertensive disorders, and ranked two as the leading cause of death [4]. In Indonesia, the Indonesian Health Demographic Survey (2013) estimated maternal mortality due to hypertensive disorders as around 27.1% [5]. The cause of preeclampsia is not yet known clearly. Many theories have been suggested, but there is no definitive cause postulated. Preeclampsia was caused by placental hypoxic/ischemic and other oxidative stress causing an endothelial dysfunction. The soluble factors discharged from ischemic placental to maternal serum have an important role in causing endothelial dysfunction, which characterized this complication [6].
Endothelial dysfunction in preeclampsia is caused by anti-angiogenic condition which is characterized by the large value of soluble FMS-like thyrosinkinase-1 (sFlt-1) and soluble endoglin, together with lower value of Placental Growth Factor (PGF) and VEGF [7]. Part of VEGF has an important role in regulating cell functions (such as proliferation, migration, survival, and permeability) which occurs during development of vascular and angiogenesis in physiologic or pathologic circumstances through its binding to several receptors. VEGFR-2 is a main mediator of VEGF-A pathologic effects, involved in the migration and cellular survival [8]. VEGF-A is also responsible in differentiating cytotrophoblast to become multinuclear syncytiotrophoblast or extravillous trophoblast [8,9]. Extravillous trophoblast has an important role in developing uteroplacental circulation. Oxygen deprivation in this stage may cause placental hypoxia [9]. Another factor which seems to be involved in this process is 2-ME. 2-ME may inhibit development, angiogenesis, and VEGF expression [10].
2-ME is an estradiol metabolite produced with the help of Catechol-O-Methyltransferase (COMT) [11]. COMT is an enzyme mostly expressed in the liver and kidney, and it participates in norepinephrine clearance and circulation homeostasis [12,13]. Serum 2-ME rises steadily during pregnancy, and reaches plateau during the last trimester, however 2-ME is suppressed in women with preeclampsia. In oxygen deprivation, 2-ME induces cytotrophoblast differentiation into invasive endovascular phenotype. At 8-10 weeks of pregnancy, the placenta will sustain from hypoxia (O2 pressure at 3%, 15-20 mmHg). However, when lower oxygen pressure happens due to endovascular trophoblast blockage of the spiral arteries, it inhibits oxygenated maternal blood influx. 2-ME maintains placental homeostatis throughout pregnancy by cytotrophoblast differentiation and function during the low oxygen pressure [11]. In preeclampsia, hypoxia induces the stabilization of Hypoxia Inducible Factor (HIF-1 alpha), a transcription factor due to hypoxia condition, included in it is Sflt-1. 2-ME inhibits the stabilization of HIF-1 alpha, and causes the degradation and inhibition of HIF-1 alpha [12–14]. Lower COMT produces less 2-ME, thus lowers its activity in severe preeclampsia. In this study, we aimed to compare the 2-ME in women with severe preeclampsia and normotensive pregnancy to gain a better understanding of the pathogenesis of preeclampsia, and a possible underlying mechanism for therapeutic implementation, hence decreasing the morbidity and mortality rate due to preeclampsia.
Materials and Methods
A Cross-sectional study was done to examine the 2-ME difference in severe preeclampsia and normotensive pregnant patient who agreed to participate in the study. The study was conducted in Obstetrics and Gynecologic Department, from January 2013 to March 2014.
This study was approved by the Director/Ethical Medical Commission of General Hospital, in conjunction with the Faculty of Medicine, Sam Ratulangi University, Manado, Indonesia.
The inclusion criteria were pregnant women with gestational age ≥28 weeks, systolic/diastolic blood pressure of ≥160/110 (severe preeclampsia group), proteinuria (+1 dipstick or more) [15], normotensive pregnancy (as control group), and singleton alive foetus. The exclusion criteria were women with chronic medical conditions such as diabetes mellitus, asthma, and chronic renal failure, also those who were not voluntarily participating in the study. Total sample was calculated based on equation on two variable correlations; n = Zα − Zβ0.51n1+ r1− r2+3, with α: 0.05, power: 0.80, and r: 0.40, rounded up into 80 women for two groups. A blood sample was collected from patients who agreed to participate in the study. The subjects were divided into two groups. Group 1: Normotensive (n=40), Group II: Severe preeclampsia (n=40). The level of 2-ME was examined in the maternal blood serum. An amount of 5 cc of patient blood was taken and sent to laboratory to be examined with the ELISA 2-ME kit (Cayman). It was based on a competitive assay between free 2-ME and 2-ME tracer for a limited number of rabbit antiserum 2-ME specific binding sites. The result was expressed in pg/ml.
Statistical Analysis
The descriptive data and the two-group categorical data were compared using t-test, Pearson Chi-Square & Univariant Analysis using the SPSS application (Statistical Package for Sciences Solutions) version 22.
Results
[Table/Fig-1] showed that age, parity, time of onset (gestational age), newborn outcome, and birth weight did not show any significant difference (p>0.05) between the two groups. Whereas in early BMI (prepregnancy), history of preeclampsia in the past pregnancy, and delivery method, there were statistically significant differences (p<0.05)
Characteristics of the two groups.
| Characteristics | Pregnancy | p-value |
|---|
| Normotensive pregnancy | Severe Preeclampsia |
|---|
| Age | <20-year-old | 4 | 3 | 0.161 |
| 20-35-year-old | 30 | 26 |
| >35-year-old | 6 | 11 |
| Parity | Primi | 20 | 16 | 0.262 |
| Multi | 20 | 24 |
| Onset (gestational age) | <34 weeks | 3 | 3 | 0.662 |
| ≥34 weeks | 37 | 37 |
| Early BMI | Normal | 31 | 18 | <0.001 |
| Overweight | 9 | 14 |
| Obese | 0 | 8 |
| History of preeclampsia | No | 40 | 33 | 0.003 |
| Yes | 0 | 7 |
| Delivery method | Vaginal | 31 | 20 | 0.005 |
| C-section | 9 | 20 |
| Birth weight | <2.5 kg | 4 | 6 | 0.252 |
| 2.5-4 kg | 35 | 32 |
| >4 kg | 1 | 2 |
| Outcome (newborn) | Healthy | 32 | 32 | 0.407 |
| Asphyxia | 7 | 6 |
| IUFD | 1 | 2 |
[Table/Fig-2] showed patients with overweight and obese had a higher risk of severe preeclampsia who were 4.21 times higher than the group with normal BMI. Based on the delivery method, severe preeclampsia group has a higher risk of undergoing C-section (about 3.45 times).
Patient characters correlation analysis.
| Variables | Normotensive pregnancy | Severe Pre- eclampsia | p | OR | CI (95%) |
|---|
| Age (years) |
| • <20 | 4 | 3 | 0.6 | 0.41 | 0.07-2.47 |
| • 20-35 | 30 | 26 |
| Age |
| • 20-35 year | 30 | 26 | 0.18 | 0.47 | 0.29-3.73 |
| • >35 year | 6 | 11 |
| Gravidity |
| • 1 / >3 | 18 | 20 | 0.65 | 1.22 | 0.51-2.94 |
| • 2-3 | 22 | 20 |
| Parity |
| • 1 / >3 | 10 | 7 | 0.41 | 0.64 | 0.22-1.88 |
| • 2-3 | 30 | 33 |
| Pre-pregnancy BMI |
| Normal | 31 | 18 | 0.002* | 4.21 | 1.598-11.09 |
| Over/Obese | 9 | 22 |
| History of Preeclampsia |
| • No | 40 | 33 | 0.04 | 0.45 | 0.31-3.29 |
| • Yes | 0 | 7 |
| History of smoking |
| • No/Passive | 39 | 34 | 0.048* | 6.88 | 0.79-60.06 |
| • Active | 1 | 6 |
| Delivery Method |
| • Vaginal | 31 | 20 | 0.005* | 3.45 | 1.31-9.06 |
| • C-section | 9 | 20 |
| Birth Weight |
| • < 2500 gm | 36 | 34 | 0.249 | 0.6 | 0.16-2.43 |
| • ≥ 2500 gm | 4 | 6 |
| Outcome (newborn) |
| Intrauterine Foetal Death/Asphyxia | 8 | 8 | 0.5 | 1 | 0.334-2.99 |
| Healthy | 32 | 32 |
* p < 0.05
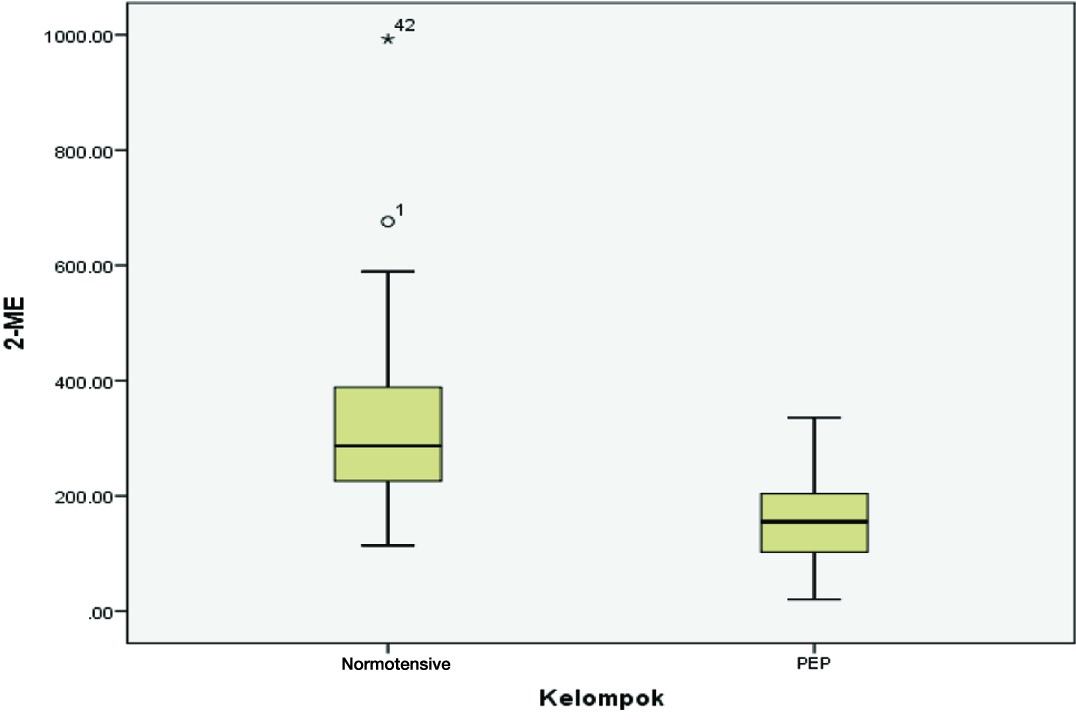
[Table/Fig-3] showed there were significant differences between systolic, diastolic, maternal weight, and 2-ME groups. A lower 2-ME was found in the severe preeclampsia group (mean 154.90+75.83 pg/ml), compared to the normotensive (mean 324.08+168.72 pg/ml).
Analysis of systolic, diastolic, maternal weight, birth weight, and 2-ME in the two groups. #in normotensive pregnancy in our center, the platelet count is not routinely checked.
| Variable | Normotensive Pregnancy | Severe Preeclampsia | 95% CI | p |
|---|
| Systolic (mmHg) | 114.50±6.78 | 168.25±12.79 | -58.305-49.195 | <0.001* |
| Diastolic (mmHg) | 75.75±5.01 | 111.50±4.27 | -37.821-33.679 | <0.001* |
| Platelet count (/mm3) | -# | 241050±88162.6 | 212854.2 -269245.77 | NA |
| Maternal Weight (kg) | 51.85±7.50 | 61.28±10.57 | -13.504-5.346 | <0.001* |
| Birth Weight (gr) | 3137.50±522.79 | 3042.50±729.20 | -187.44-377.44 | 0.252 |
| 2-ME (pg/ml) | 324.08±168.72 | 154.90±75.83 | 110.95-227.41 | <0.001* |
*p < 0.05
[Table/Fig-4] showed there was no significant difference in the 2-ME based on the difference of age of gestation (onset), parity, and pre-pregnancy BMI in both groups.
Comparison of 2-ME both groups based on the age of gestation (onset), parity, and prepregnancy BMI.
| 2-ME Value | p-value | CI |
|---|
| Normotensive | Severe Preeclampsia |
|---|
| Gestational Age/ Onset |
| * <34 weeks/ Early onset | 399.90±239.14 | 161.43±38.13 | 0.5 | 202.79-314.03 |
| * >34 weeks/Late onset | 317.93±164.75 | 154.37±78.39 |
| Parity |
| * Primi | 336.38±198.67 | 166.03±63.64764 | 0.9 | 210.38-269.81 |
| * Multi | 310.48±132.10 | 147.48±83.46 |
| Pre Pregnancy BMI |
| *Normal | 346.11±178.75 | 164.34±70.29 | 0.21 | 194.70-258.21 |
| *Overweight/ Obese | 248.18±102.97 | 147.18±80.87 |
| Average | 324.08±168.72 | 154.90±75.83 |
[Table/Fig-5] showed 2-ME was lower in severe preeclampsia (mean 154.90±75.83 pg/ml) compared to the normotensive (mean 324.08±168.72 pg/ml).
Comparison of 2-ME normotensive and severe preeclampsia pregnancy (PEP).
Discussion
Numerous studies suggested severe preeclampsia often developed at a certain age and parity groups. Jasovic-Siveska et al., mentioned preeclampsia often found in early primiparity and multiparity at late age group [16], while Conde-Agudello et al., found the risk of maternal age ≥35 year as 1.67 (95% CI 1.58-1.77) [17]. In this study, we did not find a significant difference in the risk of developing preeclampsia based on maternal age and parity. Skjaerven et al., mentioned that pregnancy interval also had a role. Pregnancy interval > 10 year had a risk nearly the same as the nullipara as 1.12 times per year [18].
There was a significant difference in the early BMI/pre-pregnancy and history of preeclampsia, even though there may be a bias because the data was collected by anamnesis. Getahun et al., mentioned the incidence of preeclampsia in second pregnancy was 2% with an increase of incidence along with an increase of BMI [19]. Ovesen et al., mentioned a higher risk of preeclampsia as much as 1.9 in an overweight BMI group, followed by 3 and 4.4 for obesity and severe obesity respectively [20], while Getahun et al., mentioned an increase risk of preeclampsia at more than 5 times if the BMI increases from underweight in pre-pregnancy to obese in the pregnancy, two times from normal to overweight, and 3.7 times from overweight to obese. Nonetheless, the risk of developing preeclampsia still persisted in African-American ethnic group, even with a reduction of BMI from obese or overweight to normal [19].
In this study, the 2-ME was lower in the severe preeclampsia group. This result resembled the early study of Kanasaki et al., in which samples were limited, yet it showed a lower 2-ME in preeclampsia [21]. Our study showed the average of 2-ME at term pregnancy was 324.08±168.72 pg/ml in normotensive group and 154.9±75.83 pg/ml in severe preeclampsia, showing a significant difference. There was no significant difference between the two groups in terms of maternal age, parity, and gestational age. The sample proportion of both groups was nearly the same, suggesting that 2-ME was not affected by these variables. 2-ME seems to have a role in reducing the risk of developing preeclampsia by cardiovascular and renal protection, and its anti inflammatory effect [21].
Lee et al., mentioned that in oxygen deprivation, 2-ME induced trophoblastic invasion into extracellular matrix [11]. At first trimester, when the foetus is rapidly growing, hypoxic condition is common. Along with pregnancy progression, hypoxaemia is supposed to regress as the foetal vascular development slows down. Nevertheless, in preeclampsia, the hypoxaemia persists up to third trimester [22].
In a normal condition, HIF-1alpha, a subunit of a transcription factor HIF-1, is activated during hypoxaemia to transcript genes which have a role in angiogenesis, oxygen transport, glucose metabolism, growth hormone signalling, apoptosis, and metastasis and invasion [11,23]. Apparently, HIF-1alpha is one of the molecular targets of 2-ME. It was proven in a clinical trial where pregnant rats with COMT deficiency and placental hypoxaemia presented a regulated form of HIF-1alpha, TGF-beta3, and TIMP-2 [11].
The increased COMT activity will also increase the hydroxy-estrogen development into methoxy-estrogen, so that the surrounding becomes more estrogenic. COMT will increase in the early period of third trimester and reach its peak at parturition. However, while estrogen production is done by the foetal membrane, the COMT activity pathway is related to the uterine contractility [24]. A lower 2-ME indicates that COMT did not produce enough amount of 2-ME and the pregnancy may develop into preeclampsia. On the contrary, a sympathetic activation causing an increase of catecholamine in the blood circulation, along with a vascular constriction, is also present in preeclampsia. Catecholamine competes with catecholestradiol in inducing methylation of COMT, which causes an interference in 2-ME metabolism [12].
In some research on preeclampsia, the placenta developed an over expression of anti angiogenic factors like sFlt-1, thus inducing symptoms of preeclampsia in pregnant rats [25]. According to Dragun et al., injection of 2-ME will reduce the HIF-1alpha expression and inhibit the sFlt-1 [12]. 2-ME protective effect is expected to recover the balance between the pro and anti angiogenic factors. When pregnant rats were given 2-ME inhibitor, they developed preeclampsia. Pertegal et al., mentioned that 2-ME value has a significant negative correlation with sFlt-1 and a positive correlation with PIGF, in which women in control group had high 2-ME [26].
Our previous study showed that low 2-ME in the second trimester caused an increase of sFlt-1 in the third trimester. This negative correlation showed that 2-ME deficiency may cause an increase of sFlt-1, even though it did not prove that the hypoxic condition represented by HIF-1alpha was temporary [21].
There was some evidence contending the statement that 2-ME and COMT only correlate with early onset preeclampsia. Lai et al., who studied 15 normal term pregnancy and 15 term preeclampsia, concluded that the placental COMT expression regression was also correlated with term preeclampsia [27].
Several studies showed a great concern about the COMT correlation with numerous risk factors, preceding 2-ME. Lim et al., mentioned that maternal COMT gene homogenicity increased the susceptibility to severe preeclampsia [28], but Hill et al., did not prove a significant correlation between COMT and maternal BMI [29].
Gene defect in COMT and its impact on 2-ME was also reported to be related to the higher risk of recurrent preeclampsia in certain population [30].
Hormones and its metabolites have long known to be correlated with pregnancy. Jobe et al., tried to describe the estrogen and its metabolites in maternal circulation in preeclampsia. Even though it was in an early phase, they explained the possibility of synthesis aberration, metabolism, and accumulation of plasma estrogen and its metabolites in preeclampsia patient circulation, which might be correlated with the vascular defects [31].
In summary, this study provides initial data showing 2-ME value is lower in severe preeclampsia than normotensive pregnancy. It also supports the hypothesis of 2-ME has a role in the pathogenesis of preeclampsia. Thus, 2-ME can be studied more thoroughly for its therapeutic implementation.
Limitation
Since, it was a cross-sectional study we could not measure the progression of the condition. The pre-pregnancy BMI data were collected by anamnesis only, not by standardized measurement. Due to the multifactorial aetiologies of preeclampsia, it is susceptible to bias.
Conclusion
2-ME in severe preeclampsia was lower than in a normotensive pregnancy. It means that COMT produced insufficient amount of 2-ME. 2-ME seemed to have a role in reducing the risk of developing preeclampsia by its cardiovascular and renal protection, and anti-inflammatory effect. It appeared to be a component that has a role in the pathogenesis of preeclampsia.
* p < 0.05
*p < 0.05