Maternal and Cord Blood Plasma sEng and TGF-β1 in Patients with Hypertensive Disorders of Pregnancy: A Pilot Study in a South Indian Population
Vickneshwaran Vinayagam1, Zachariah Bobby2, Syed Habeebullah3, Latha Chaturvedula4, Shruthi K Bharadwaj5
1 Ph D Scholar, Department of Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India.
2 Professor and Head, Department of Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India.
3 Professor, Department of Obstetrics and Gynaecology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India.
4 Professor, Department of Obstetrics and Gynaecology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India.
5 Student (DM Neonatology), Department of Neonatology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Zachariah Bobby, Professor and Head, Depatment of Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry 605006, India.
E-mail: zacbobby@yahoo.com
Introduction
Hypertensive Disorders of Pregnancy (HDP) are one of the most widespread complications of pregnancy that affects both mother and foetus. It has been observed that in Preeclampsia, the release of soluble angiogenic factors from the ischemic placenta into maternal plasma plays a crucial role in the pathogenesis.
Aim
To assess the plasma Soluble Endoglin (sEng) and Transforming Growth Factor (TGF-β1) levels in various types of HDP and to correlate the levels of these markers with the pregnancy outcome.
Materials and Methods
A total of 128 pregnant women were recruited and the study was carried out for a period of three years. Cord blood and maternal blood plasma levels of sEng and TGF-β1 were analysed by ELISA kits in Control Pregnant Women (CPW), Gestational Hypertension (GH), Early Onset Preeclampsia (EOPE), Late Onset Preeclampsia (LOPE), and Eclampsia (E) during third trimester. The Gestational Age (GA) at the time of delivery and Birth Weight (BW) of the baby also were also evaluated.
Results
The circulating levels of maternal and cord blood sEng were significantly higher in EOPE and E compared to CPW and GH. However, the maternal and cord blood levels of TGF-β1 were significantly lower in LOPE and E when compared to CPW and GH. The GA and BW of the baby were found to be significantly lower in EOPE and E compared to CPW, GH and LOPE. Also, a negative correlation was observed between sEng levels with pregnancy outcome; GA and BW. And also, a positive correlation was found between TGF-β1 and pregnancy outcome.
Conclusion
A generalised angiogenic imbalance and poor birth outcomes were observed in HDP. There is a spectrum of biochemical derangements related to angiogenesis in GH, EOPE, LOPE and E.
Angiogenic markers, Eclampsia, Preeclampsia
Introduction
HDP are one of the most widespread complications of pregnancy that affects 5-10% of pregnancies [1]. As per the National High Blood Pressure Education Program (NHBEP), HDP has been classified into GH, Preeclampsia (PE), E, PE superimposed on chronic hypertension and chronic hypertension [2]. PE is a pregnancy disorder that is frequently associated with the maternal and foetal morbidity and mortality [3]. However, the studies on E are very limited. About 10-15% of maternal mortality is due to PE-E in developing/underdeveloped countries [4]. In more than 15% of the cases, the premature delivery imparts a burden on the foetus. Information on HDP is limited other than PE. It has been observed that in PE, the release of soluble angiogenic factors such as sFlt-1 from the ischemic placenta into maternal plasma plays a crucial role in the endothelial dysfunction [5]. An imbalance between pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), and anti-angiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1) and soluble endoglin (sEng), has been observed before the onset of PE and after the clinical diagnosis [5,6]. This may also be a major factor for the future development of cardiovascular risk in the baby and the mother of HDP [7]. Endoglin (Eng) or CD105, a transmembrane glycoprotein localized on cell surfaces functions as a coreceptor for transforming growth factor TGF-β1 and TGF-β3 isoforms [8]. Soluble endoglin, an antiangiogenic factor that binds to circulating TGF-β1 ligand prevents its availability to the cellular downstream proangiogenic and vasodilatory effect in PE [9].
In our study, we attempted to assess the plasma levels of sEng and TGF-β1 of cord blood and maternal blood in various types of HDP and also to correlate them with their pregnancy outcome (Gestational age (GA) and Birth weight (BW) of the baby).
Materials and Methods
The study subjects were recruited from the Department of Obstetrics and Gynaecology, Women and Children Hospital, JIPMER, India. The study was carried out after getting the approval (JIP/IEC/1/2012/7) from the Institute ethics committee (Human). It was a cross-sectional study and was carried out for a period of three years from Jan 2013 to Dec 2015. The sample size was calculated to be 95 (19 in each group) using open epi progamme, with mean and Standard Deviation (SD) of TGF-β1 among PE and control [10]. However, we have increased the sample size to 128 for better results. Out of this 128 study subjects, 42 were normotensive pregnant women and 86 were HDP. The HDP cases were further divided into four groups [2,11] as GH-27, EOPE – 21, LOPE - 20, and E-18. GH is the pregnancy disorder where the pregnant women are first diagnosed with hypertension after 20 weeks of gestation without proteinuria. EOPE is a condition where the onset of hypertension (≥140/90 mm/Hg) and proteinuria (>300mg/day) occurs between 20-34 weeks of gestation in previously normotensive non proteinuric pregnant women. LOPE is a condition where the onset of PE is after 34 weeks of gestation. E is a condition that has features of PE along with seizures/coma that happen during pregnancy but are not due to preexisting or organic brain disorders. Primi pregnant women with HDP (case) and without any complication (control) of age group 18-30 were included in the study. Study subjects with clinical complications like gestational diabetes, chronic hypertension, and preeclampsia superimposed on chronic hypertension, multipara, major congenital anomalies, kidney disease infection in current pregnancy and autoimmune disorders were excluded from the study. After obtaining an informed consent from all the study subjects, 5 ml of fasting venous blood and cord blood (after delivery) was collected; plasma was separated by centrifugation at 3500 rpm for 10 min and stored immediately at -40°C until analysis. sEng (Quantikine, R and D systems, Minneapolis, MN, USA) and TGF-β1 (DRG-International, USA) were analysed using commercially available ELISA kits as per the manufacturer’s instructions.
Statistical Analysis
All the values are expressed as mean±S.D and analysed using Statistical Package for Social Sciences (SPSS), version 19.0 software. Normality of the data was done by Kolmogorov-Smirnov and Shapiro-Wilk test. Analysis of variance (ANOVA) was carried out to compare more than two mean and as they came out to be statistically significant, Tukey’s post-Hoc test was carried out to make pairwise group comparisons [Table/Fig-1a,b].
| Sum of Squares | df | Mean Square | F | Sig. |
|---|
| Birth weight of the baby (kg) | Between Groups | 55.139 | 4 | 13.785 | 54.917 | .000 |
| Gestational Age at birth (days) | 42072.603 | 4 | 10518.151 | 59.519 | .000 |
| sENG-MB | 18374.652 | 4 | 4593.663 | 35.214 | .000 |
| sENG-CB | 361.524 | 4 | 90.381 | 2.730 | .032 |
| TGFB1-MB | 1936.080 | 4 | 484.020 | 5.154 | .001 |
| TGFB1-CB | 3163.091 | 4 | 790.773 | 7.638 | .000 |
Demographic details and the levels of plasma sEng and TGF-β1 in hypertensive disorders of pregnancy.
| Demographic Details and Plasma Markers | Control Pregnant women | Hypertensive disorders of pregnancy (n=86) |
|---|
| Gestational Hypertension | Late onset preeclampsia | Early onset preeclampsia | Eclampsia |
|---|
| Age (years) | 23.7±2.9 | 23.77±2.76 | 22.76±2.77 | 23.9±2.77 | 21.88±1.9 |
| Gestational Age at birth (days) | 270.9±7.12 | 272.5±6.8 | 265,3±9.6 | 230.6±20.8 abc | 230.2±21.5 abc |
| Birth weight of the baby (kg) | 2.9±0.35 | 2.88±0.43 | 2.58±0.55 | 1.47±0.65 abc | 1.32±0.60 abc |
| sEng (MB) (ng/ml) | 38.74±14.99 | 34.95±10.12 | 56.12±12.88 | 64.33±3.2 ab | 62.5±6.9 ab |
| sEng (CB) (ng/ml) | 15.22±2.96 | 13.17±2.15 | 17.26±11.68 ab | 17.17±4.88 ab | 18.0±4.4 ab |
| TGF-β1 (MB) (ng/ml) | 23.3±9.48 | 23.74±13.71 | 15.4±8.2 ab | 16.25±5.49 | 15.17±8.15 ab |
| TGF-β1 (CB) (ng/ml) | 37.63±7 | 26.32±13.84 a | 26.32±13.84 a | 32.58±9.0 | 27.2±8.1 a |
a: versus Control pregnant women, p< 0.05
b: versus gestational hypertension (GH) women, p< 0.05
c: versus Late onset preeclampsia (LOPE) women, p< 0.05
sEng: Soluble Endoglin; TGF- β1: Transforming growth factor beta 1;
MB- Maternal blood; CB: Cord blood.
Tukey’s Post-Hoc Test
Pearson’s correlation was employed to assess the correlation between the plasma angiogenic marker, sEng and its ligand TGF-β1 with pregnancy outcome; GA at delivery and BW of the baby. Statistical significance was assumed if a null hypothesis could be rejected at a p-value of ≤ 0.05
Results
No significant difference was found in the mean age of the study subjects. It has been found that the mean GA and BW of the baby at delivery were significantly lower in EOPE and E when compared to CPW, GH and LOPE. [Table/Fig-1b] shows the demographic details and the plasma levels of sEng and TGF-β1 in all the five groups.
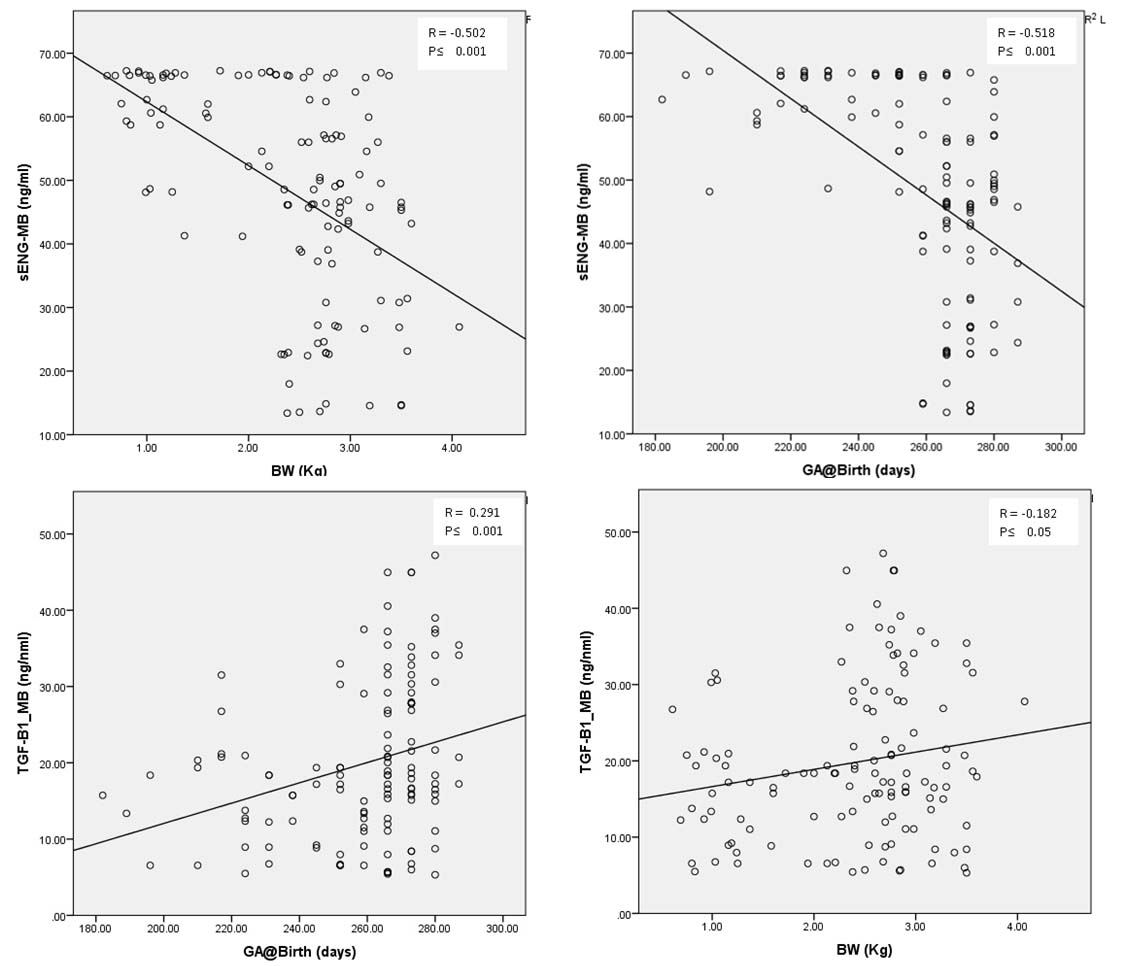
It has been found that the circulating maternal and cord blood levels of sEng were significantly higher (p < 0.05) in EOPE and E compared to CPW and GH. In addition, the cord blood values of sEng were significantly higher (p < 0.05) in LOPE compared to CPW. Similarly, the values of TGF-β1 were significantly lower (p < 0.05) in LOPE and E when compared to CPW in cord blood and also in maternal blood in GH. Besides, the values of cord blood TGF- β1 were significantly lower in GH when compared to CPW. [Table/Fig-2] shows a significant negative correlation of sEng levels of maternal blood with pregnancy outcome as assessed by GA and BW of the baby. Similary, a significant positive correlation was observed with TGF-β1 levels of the maternal blood with the pregnancy outcome. [Table/Fig-3a-d] shows the negative correlation of sEng and positive correlation of TGF-β1 with the BW of the baby and GA at delivery respectively.
Correlation between maternal blood sEng and TGF β1 with gestational age and birthweight of the baby.
| Gestational Age at Birth | Birth weight |
|---|
| Pearson’s coefficient (r) | p- value | Pearson’s coefficient (r) | p-value |
|---|
| sEng (MB) | -0.518 | < 0.001 | -0.502 | < 0.001 |
| TGF-β1 (MB) | 0.291 | < 0.001 | 0.182 | < 0.043 |
Correlation of sEng(MB) with the birthweight (BW) of the baby (a), and Gestational age (GA) at the time of delivery (b); Correlation of TGF-b1(MB) with the GA at the time of delivery (c) and and BW of the baby (d).
Discussion
HDP is a pregnancy disorder where there is a poor microvascular development of feto-placental unit due to shallow trophoblast invasion and insufficient spiral artery remodeling. This leads to an ischemic/inadequate blood supply to the foetus due to peripheral vasoconstrictions of placental beds leading to maternal and foetal hypoperfusion [7,12,13].
Endoglin is a proangiogenic factors that is an essential component of the endothelial nitric oxide sythase (eNOS) regulating vascular tone [14,15]. sEng is a truncated form of endoglin, a cell surface receptor for TGF-β that binds and antagonize TGF-β [16].
In this study, we have made an attempt to assess the relationship of circulating levels of sEng and TGF-β1 in different types of HDP. To the best of our knowledge, this is the first study, where we have classified the HDP into GH, LOPE, EOPE and E to bring out the differences in the levels of these markers in both maternal and cord blood among these groups and correlated with the GA at delivery and BW of the baby.
We have found an increase in the cord blood and maternal blood plasma levels of sEng in LOPE and E when compared to CPW and also GH. These findings have been supported by recent studies on maternal plasma of PE patients [10,17,18]. There are reports that an elevated level of sEng impairs the NO generation and activates endothelin-1 signaling leading to hypertension and maternal endothelial dysfunction [4]. We have also found an elevated ET-1 levels in our earlier study in PE compared to CPW [19]. In our study, we have also found a significant decrease in TGF-β1 levels in both maternal and cord blood plasma of LOPE and E when compared to the control as evidenced by Lim et al in the maternal circulation of PE [10]. Previous study has found an increase in the plasma levels of TGF-β1 in PE when compared to control [20]. However, no significant difference in serum TGF-β1 value was observed in an another study between PE and control group [18]. The possible mechanism could be that the loss of the angiogenic factor TGF-β1 by binding to sEng is minimal as the sEng may increase in the late onset of the disease (after 34 weeks) unlike the EOPE. There are evidences that the activated platelets are the source of circulating TGF-β1 [21]. We have found in our earlier study, elevated plasma levels of platelet derived microparticles which may be due to increased destruction of platelets [19].
We have also found a negative correlation of sEng with the pregnancy outcome as evidenced by GA and BW of the baby at the time of delivery. Also, we have found a positive correlation between TGF-β1 and the pregnancy outcome.
Hence, assessing the plasma values of these markers may play a vital role as diagnostic markers in predicting HDP, especially the severe forms; EOPE / E.
Limitation
We estimated the angiogenic marker with less sample size. The patients were treated with antihypertensive drugs which may interfere with the marker levels. Also, we could not assess the values of these angiogenic parameters in the early trimesters.
Conclusion
A generalized angiogenic imbalance and poor foetal outcomes were observed in hypertensive disorders of pregnancy. Also, there is a negative correlation found between angiogenic factor, sEng and the pregnancy outcome.
a: versus Control pregnant women, p< 0.05
b: versus gestational hypertension (GH) women, p< 0.05
c: versus Late onset preeclampsia (LOPE) women, p< 0.05
sEng: Soluble Endoglin; TGF- β1: Transforming growth factor beta 1;
MB- Maternal blood; CB: Cord blood.
Tukey’s Post-Hoc Test
[1]. Lo JO, Mission JF, Caughey AB, Hypertensive disease of pregnancy and maternal mortalityCurr Opin Obstet Gynecol 2013 25(2):124-32. [Google Scholar]
[2]. Report of the national high blood pressure education program working group on high blood pressure in pregnancyAm J Obstet Gynecol 2000 183(1):S1-S22. [Google Scholar]
[3]. Shembrey MA, Noble AD, An instructive case of abdominal pregnancyAust N Z J Obstet Gynaecol 1995 35(2):220-21. [Google Scholar]
[4]. Duley L, Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the CaribbeanBr J Obstet Gynaecol 1992 99(7):547-53. [Google Scholar]
[5]. Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ, Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsiaBr J Obstet Gynaecol 1999 106(10):1019-22. [Google Scholar]
[6]. Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Soluble endoglin contributes to the pathogenesis of preeclampsiaNat Med 2006 12(6):642-49. [Google Scholar]
[7]. Eiland E, Nzerue C, Faulkner M, PreeclampsiaJ Pregnancy 2012 2012:586578 [Google Scholar]
[8]. Barbara NP, Wrana JL, Letarte M, Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamilyJ Biol Chem 1999 274(2):584-94. [Google Scholar]
[9]. Perucci LO, Gomes KB, Freitas LG, Godoi LC, Alpoim PN, Pinheiro MB, Soluble endoglin, transforming growth factor-Beta 1 and soluble tumor necrosis factor alpha receptors indifferent clinical manifestations of preeclampsiaPLoS One 2014 9(5):e97632 [Google Scholar]
[10]. Lim JH, Kim SY, Park SY, Lee MH, Yang JH, Kim MY, Soluble endoglin and transforming growth factor-beta1 in women who subsequently developed preeclampsiaPrenat Diagn 2009 29(5):471-76. [Google Scholar]
[11]. Von Dadelszen P, Magee LA, Roberts JM, Subclassification of preeclampsiaHypertens Pregnancy 2003 22(2):143-48. [Google Scholar]
[12]. Gilbert JS, Nijland MJ, Knoblich P, Placental ischemia and cardiovascular dysfunction in preeclampsia and beyond: Making the connectionsExpert Review of Cardiovascular Therapy 2008 6:1367-77. [Google Scholar]
[13]. Lecarpentier E, Tsatsaris V, Chronic hypertension and pregnancyRevue Du Praticien 2012 62(7):924-25.:921-22. [Google Scholar]
[14]. Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, CD105 prevents apoptosis in hypoxic endothelial cellsJ Cell Sci 2003 116(Pt 13):2677-85. [Google Scholar]
[15]. Jerkic M, Rivas-Elena JV, Prieto M, Carrón R, Sanz-Rodríguez F, Pérez-Barriocanal F, Endoglin regulates nitric oxide-dependent vasodilatationFASEB J 2004 18(3):609-11. [Google Scholar]
[16]. Maynard SE, Karumanchi SA, Angiogenic factors and preeclampsiaSemin Nephrol 2011 31(1):33-46. [Google Scholar]
[17]. Shahul S, Medvedofsky D, Wenger JB, Nizamuddin J, Brown SM, Bajracharya S, Circulating antiangiogenic factors and myocardial dysfunction in hypertensive disorders of pregnancyHypertension 2016 67(6):1273-80. [Google Scholar]
[18]. Perucci LO, Gomes KB, Freitas LG, Godoi LC, Alpoim PN, Pinheiro MB, Soluble endoglin, transforming growth factor-Beta 1 and soluble tumour necrosis factor alpha receptors indifferent clinical manifestations of preeclampsiaPLoS One 2014 9(5):e97632 [Google Scholar]
[19]. Vinayagam V, Bobby Z, Habeebullah S, Chaturvedula L, Bharadwaj SK, Plasma markers of endothelial dysfunction in patients with hypertensive disorders of pregnancy: A pilot study in a South Indian populationJ Matern Fetal Neonatal Med 2016 29(13):2077-82. [Google Scholar]
[20]. Peraçoli MT, Menegon FT, Borges VT, de Araújo Costa RA, Thomazini-Santos IA, Peraçoli JC, Platelet aggregation and TGF-beta(1) plasma levels in pregnant women with preeclampsiaJ Reprod Immunol 2008 79(1):79-84. [Google Scholar]
[21]. Lev PR, Salim JP, Marta RF, Osorio MJ, Goette NP, Molinas FC, Platelets possess functional TGF-beta receptors and Smad2 proteinPlatelets 2007 18(1):35-42. [Google Scholar]