Hans Berger was the first scientist to demonstrate recording of electrical activity over human scalp which lead to the most important discovery of the millennium in the field of neurophysiology, the electroencephalography [1]. Yet another important landmark was the discovery of ophistochronic averaging technique by Kornhuber and Deecke enabling the scientific world to record cortical potentials prior to movement, known as the BP or readiness potentials [2]. These potentials represent the cortical activity involved in planning and execution of movement [3]. Principle sources of these potentials lies in supplementary motor cortex and primary motor cortex of the brain [4]. Thus, recording cortical potentials prior to movement has been a good non invasive tool to study motor cortices in disease, especially in PD. PD is a progressive neurodegenerative disorder affecting the motor system of the body characterized by tremors at rest, rigidity, bradykinesia, and gait impairment. It is the second commonest form of neurodegenerative disorder with prevalence of 0.3% in general population [5]. The loss of dopaminergic neurons in the substantianigra leads to imbalance in the basal ganglia circuitry and subsequent excessive inhibition of motor circuit [6]. Apart from the rate model, another explanation for pathophysiology in PD is abnormal synchronization in structures of basal ganglia leading to disruption of cortical activity necessary for movement [7]. The principle source of BP is the supplementary motor cortex which receives predominant input from basal ganglia [8]. Hence, researches have studied the effect of the disease on BP.
Waveform observations of BP have demonstrated two distinguishable components, namely the early and late component. Topographic distribution of the potentials shows that the early component is bilaterally symmetrical and contributed by bilateral activation of supplementary motor cortex [4,9]. At about 0.5 seconds prior to the onset of movement the slope of waveform steeps up marking the presence of second or the late component of these potentials. Late component is predominantly observed over the contralateral side of the cortex with generators from contralateral supplementary and primary motor cortices [4,9]. Just before or around the movement onset the potential rises again forming the peak component principally contributed from the contralateral primary motor cortex [9]. It is the early component that has been found to be smaller in PD as compared to healthy controls [10]. Yet another study observed increased amplitude in BP when compared to age matched controls [11]. However, the contribution of basal ganglia in BP cannot be neglected as bilateral lesion of this region results in flatter BP as compared to controls [12]. Treatment with anti parkinsonian medications decreases the abnormal synchronization in basal ganglia relieving the inhibition on motor networks and providing symptomatic relief for the patients [13]. Administration of anti parkinsonian medications helps in improving the early component of BP thus improving motor planning in PD [14]. Though these medications provide some symptomatic relief in advanced stages they do not stop the progression of the disease. It remains unclear whether the anti parkinsonian medications also improve cortical activity prior to movement even in advanced stages of the disease. To explore this query we recorded scalp BP in patients with varying grades of severity with the aim to study the relationship between disease severity and various components of BP. The objectives of our study was to compare BP parameters like early slope, late slope, amplitude and post peak slope in PD with healthy age matched controls and also to compare the above parameters among patients with varying grades of severity.
Materials and Methods
Subjects
This observational study was conducted in the Cognitive Neurophysiology Laboratory, Department of Physiology, All India Institute of Medical Sciences, New Delhi, India, between November 2011 and November 2014. Study was approved by Institute Ethical Committee and in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki 2000). All patients with PD were recruited from movement disorders clinic of department of neurology. Written informed consent was obtained from all subjects. Only male patients with age between 50-70 years with PD of H&Y scale ≥ 2 were recruited. Only right handed subjects assessed using Oldfield questionnaire were included in this study [15]. The patients were subdivided into three groups according to severity of viz., mild (H&Y 2, PD1), moderate (H&Y 3, PD2) and severe (H&Y 4, PD3).
Controls were right handed healthy volunteers between the ages of 50-70 years. Subjects with history of any head trauma, other neurological or psychiatric disorders were excluded from the study. Severity of the disease was also assessed using Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores (UPDRS part III) [16]. Out of 20 patients recruited in PD3 group, only 17 could perform the motor task successfully and that also only in medication “on” state. Data from five subjects was excluded due to tremor artefacts. Based on the PD3 group equal number of age matched participants were included from other groups (PD1, PD2, controls) before including their data for statistical analysis. Each group of controls as well as cases consisted of 12 subjects [Table/Fig-1] [17].
Details of the prticipants in the study. Values represented as mean +SD, *** represents p<0.001 when compared to PD3 group, ### represents p<0.001 when compared with PD2. LED: levodopa equivalent dosage [17].
| Parameters | PD3 | PD1 | PD2 | Controls | p value |
|---|
| Numbers of subject (n) | 12 | 12 | 12 | 12 | |
| HY Scale | 4 | 2 | 3 | -- | -- |
| Age (Years) | 54.6±75.36 | 54.00±3.28 | 55.25±3.96 | 54.50±4.46 | 0.916 |
| Duration of disease (years) | 13.33±2.50 | 7.50±1.98*** | 8.92±2.43*** | -- | <0.001 |
| UPDRS III motor score | 37.92±9.92 | 13.83±4.53***### | 25.42±4.74*** | -- | <0.001 |
| LED (mg/day) | 6888±194.81 | 409.08±158.60 | 991.78±257.35 | -- | -- |
Study Paradigm
The potentials were recorded using Evoked Potential Recorder (Neuropack 8, Nihon Kohden, Japan). Potentials were recorded at Cz, C3 and C4 electrode sites placed according to international 10-20 system with linked earlobes electrodes (A1 & A2) as reference. Forehead electrode (Fpz) used as ground and Fp1/ Fp2 sites were used for detecting eye blinks. The Electromyogram (EMG) from the extensor carpi radialis muscle was used as a trigger for collection of BP. The signal was amplified with a gain of 10,000 through a filter band pass 0.05-80 Hz for scalp recordings and 0.05-3 KHz for EMG with notch filter to reduce electrical disturbance. Impedance was kept less than 5 KΩ throughout the recording.
Recording was done with participants seated on an armchair in “on” medication state. They were asked to keep their eyes open and fixed on a centre of a screen during the recording. The participants were trained to perform precise right wrist extensions (around 60 degree above horizontal position) once every 10 seconds. To ensure active participation during the task, the subjects were asked to keep the interval between the contractions random but always more than 10 seconds. They were given feedback to stop whenever the researcher observed that the contractions were rhythmic and averaging was paused. The onset of EMG signal was used as trigger for back average the EEG 3.0 seconds prior to and 1.0 second after the EMG onset. Sweeps with EMG signal lasting for more than 0.5 second, EEG amplitudes of more than 60 μV, eye blinks or other motion artefacts were excluded from the averaging. hundred such artefact free sweeps (trials) were averaged to obtain BP.
Statistical Analysis
All participants were allotted code numbers in order to blind investigator during recording and analysis. Baseline activity was calculated from -3000 ms to -2500 ms i.e., prior to the onset of BP in all recordings. The BP waveform was analysed for following parameters: peak amplitude, early slope, late slope and post peak slope was calculated as shown in [Table/Fig-2].
A representative bereitschaftspotentials record at Cz site among control group. The maximum amplitude of bereitschaftspotentials occurring near the time of movement (avound-100 ms) was noted as peak amplitude. Early slope, late slope and post peak slope was calculated as an average slope over the time period of -1500 to -600 ms, -500 ms to 0 ms and 0 ms to 500 ms relative to EMG onset using linear regression respectively.
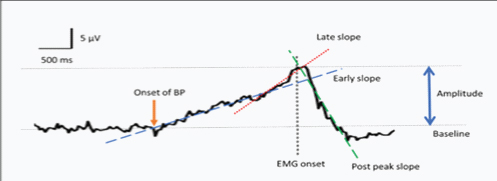
Based on normality of the data comparison between the groups was done using analysis of variance (ANOVA)/ kruskal-wallis and post-hoc analysis was done using Bonferroni multiple comparison tests. All statistical tests used were two-tailed with p<0.05 used to determine statistical significance.
Results
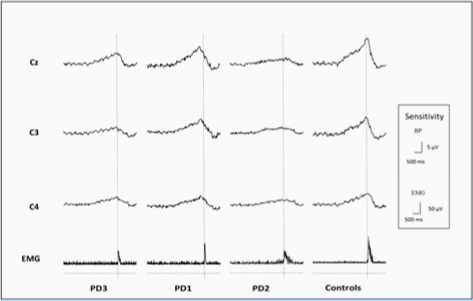
Demographic details are described in [Table/Fig-1]. Age did not defer significantly among the groups (p=0.9162). Duration of disease was significantly different among the patients, PD1 (p<0.001) and PD2 (p=0.00014) having significantly less years with disease as compared to PD3. Disease severity measured by UPDRS motor scores showed significant less scores in PD1 (p=0.00017) and PD2 (p=0.00297) as compared to PD3. UPDRS scores were also less in PD1 as compared to PD2 (p=0.00031). BP was maximum at Cz site for all groups as shown in [Table/Fig-3].
This figure shows average BP records for 1200 trials in each group at sites Cz, C3 and C4. Rectifed EMG is shown below BP records. The dotted vertical line represnts the onset of EMG signal that used as trigger for back averaging the EEG 3.0 seconds prior to and 1.0 second after the EMG onset.
BP parameters were significantly different among groups at Cz, C3 and C4 [Table/Fig-4].
Comparing a) peak amplitude; b) early slope; c) late slope and d) post peak slope among various groups. Box and whiskers plot (min to max) with central horizontal line depicting the median, vertical box depicting limits 25% and whiskers the whole range.
*p>0.05, **P<0.01 and ***p<0.001 when compared to control group #p<0.05, ##p<0.01 and ###p<0.001 when compared to PDI group. p<0.05 and p<0.001 when compared to PD2 group.
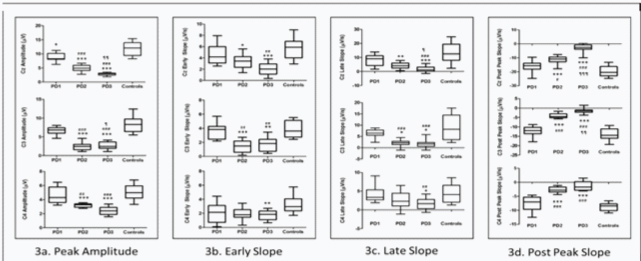
Peak amplitude at Cz was significantly less in PD1 (p=0.011), PD2 (p<0.001), PD3 (p<0.001) as compared to controls. At site C3 and C4 peak amplitude was significantly less in PD2 (p<0.0001, p=0.00058) and PD3 (p<0.001, p=0.00036) as compared to controls respectively; while amplitude at these sites was not significantly different in PD1 (p=0.53, p=0.91) as compared to controls. Peak amplitude at Cz, C3, C4 was significantly less in PD2 (p<0.001, p<0.001, p=0.00539), PD3 (p<0.001, p<0.001, p=0.0058) as compared to PD1 respectively. Peak amplitude at Cz and C3 was significantly less in PD3 (p=0.0086, p=0.028) as compared to PD2. Early slope at Cz and C3 was significantly less in PD2 (p=0.0175, p<0.001), PD3 (p<0.001, p=0.0026), but not in PD1 as compared to controls; early slope at C4 was significantly less in PD3 (p=0.0089) but not in PD1, PD2 as compared to controls. Early slope at Cz was significantly less in PD3 (p=0.0012) as compared to PD1; at C3 site early slope was less in PD2 (p=0.0011), PD3 (p=0.0016) as compared to PD1. Late slope at Cz and C3 was significantly less in PD2 (p=0.00858, p=0.027), PD3 (p=0.0002, p=0.011), but not in PD1 as compared to controls; late slope at C4 was significantly less in PD3 (p=0.0409) but not in PD1 and PD2 as compared to controls. Late slope at C3 was significantly less in PD2 (p<0.001) as compared to PD1. Late slope at Cz, C3 and C4 was significantly less in PD3 (p=0.00043, p<0.001, p=0.0086) as compared to PD1. Late slope was also less in PD3 as compared to PD2 at Cz (p=0.03336). Post peak slope was significantly more in PD2 and PD3 at Cz (p=0.00013, p<0.001), C3 (p=0.00022, p=0.00042) and C4 (p<0.001, p<0.001) compared to controls; but not in PD1 compared to controls. Post peak slope was significantly more in PD2 and PD3 as compared to PD1 at Cz (p=0.01746, p<0.001), C3 (p=0.00012, p<0.001) and C4 (p<0.001, p<0.001) sites. This post peak slope was also more in PD3 compared to PD2 at Cz (p<0.001) and C3 (0.00184).
Discussion
PD is a progressive neurodegenerative disease with basal ganglia dysfunction due to the loss of dopaminergic neurons in substantianigra. The dysfunction in basal ganglia output leads to excessive inhibitory influences on thalamo-cortical pathways [6] and thus, affecting the movement planning and execution in PD [4]. Though, dopaminergic medications help in ameliorating the symptoms in PD, they do not stop progression of disease. However, it is unclear whether these medicines improve motor planning even in advanced stages of PD. The aim of this research was to study the cortical activity prior movement i.e., BP with increasing severity of Parkinson’s disease.
As evident from the [Table/Fig-3], BP records were flatter in PD especially with increasing severity of disease. Most prominent change was observed at Cz site (vertex). Similar findings have been reported with reduction in amplitude of BP throughout the waveform development in bilateral PD [18,19]. Though the shape of BP waveform was preserved in mild severity (HY scale 2), peak amplitude was observed to be decreased at Cz site in the disease. However, with increasing severity of disease (in HY scales 3 and 4) all BP parameters were deranged when compared to controls at sites Cz and C3. Amplitude and post peak slope showed prominent differences in scales 3 and 4 compared to scale 2 at all sites. Furthermore, comparing moderate scale 3 with severe scale 4 showed decreasing amplitude, late slope and post peak slope at Cz site in the later. All these findings point to evidence of increasing defect in cortical activity during movement with increasing scales of severity in PD in spite of dopaminergic medications. This is the first study that examines various parameters of bereitschaftspotentials viz. early slope, late slope, amplitude and post peak slope with varying grades of severity using H-Y scale. Abnormal BP waveforms in PD have been reported by many studies [9,10,14], while some studies have observed near normal to increased amplitude of BP in PD compared to age match controls [11]. There can be two reasons for such discrepancies, first might be due to differences in methodology of recording BP. Early slope BP as well as peak amplitude have been observed lower in patients with PD compared to controls during self-initiated movement [9,10], similar to our recording paradigm. Indeed imaging studies have shown decreased activation of bilateral Supplementary Motor Areas (SMA) prior to movement in PD [20–22]; these are the same areas contributing maximally for early part of BP. Even recording from SMA neurons have shown two types of temporal firing patterns (about 2 seconds prior and other 0.5 seconds) before movement [23,24]. SMA plays an important role during the preparation phase before the onset of voluntary movement and co-ordinates with sub cortical structures to plan the order of movement [3,10,25]. Though the late part is contributed by contralateral primary motor cortex, studies have found that this late part of BP to be less evident in PD as compared to controls when participants were asked to freely select their movement; such observations are due to defective activation of supplementary motor cortices throughout the generation of BP [14]. Second reason for differences in BP results among the mentioned studies may be due to varying severity of PD among the participants. We found almost normal BP waveform in mild PD cases, while all parameters are deranged in severe PD cases when compared to controls. Hence, patient group homogeneity with respect to disease severity must be accounted when comparing with healthy controls. Indeed, the amplitude of BP has been found to negatively correlate with disease severity in PD [4,9]. Increasing severity of disease may result in more defects in motor planning. In our study, we found flatter BP with increasing severity of disease with low peak amplitude and less steep early, late and post peak slopes; another study has reported similar findings in late stages as compared to early stages in PD [10,26]. We found graded decrease in BP with each scale of severity, especially at the vertex (Cz) implying a gradual worsening in activation of SMA prior to movement. Indeed, a recent study has found deterioration of SMA activity with progression of PD supporting our findings [27].
We found no change in early part of BP in mild PD. Indeed, dopaminergic medications have been shown to increase early BP in PD, especially in de novo patients [14]. But these anti PD drugs do not stop progression of disease and with increasing loss of dopaminergic neurons leads to increasing defects in supplementary motor cortical activity in PD [23]. In advanced stages of PD, levodopa fails to mimic the action of endogenous dopamine as in health leading to abnormal peaks in dopamine levels and further basal ganglia dysfunction [28]. This explains the defect in early and late part of BP with increasing severity of disease. The flatter BP in scales 3 and 4 also revealed less steep post peak slope. This slope represents the termination of pre-movement activity in the cortices and is found to be affected in advanced stages [14,26]. Thus, there are defects in motor planning in PD not only in motor preparation or execution but also in termination phase of movement.
Limitation
One of the limitations of our study is inherent in the H-Y scaling system. We have classified severity as mild, moderate and severe based on H-Y scale but, as seen in UPDRS-III motor scores there does appears to be variation in severity within groups. Future studies may be required with larger sample size to study effect of disease severity on motor planning using UPDRS scores.
Conclusion
This study examines the effects of disease severity on various parameters of BP. Our findings suggest that increasing severity of PD does lead to defects in the all phases motor planning in spite of medications. External dopamine fails to mimic the endogenous dopamine pattern of action and thus, fails to correct the supplementary motor cortex dysfunction in advanced stages. Recording cortical potentials prior to movement can thus be used as a good non invasive method for studying the activity of the motor related cortices in PD with varying grades of severity. Newer techniques like deep brain stimulation and transcranial magnetic stimulation should be taken into account to understand the dynamic nature of dysfunction in supplementary motor cortices with increasing severity of PD; such inclusions in stimulation paradigm will help patients for efficient motor planning and be adequately ready to act.