Cholesteatoma is a well demarcated, non-neoplastic, temporal bone cystic lesion with extensive keratinisation. Keratoma and epidermoid cyst are other possibly more accurate names suggested to describe the same. It can be classified as congenital or acquired. Its management is often complicated by its tendency to recidivism/recurrence. Long standing cholesteatomas can be a precursor for squamous cell carcinoma. We hereby present a case of giant cholesteatoma in a 45-year-old female with radiological involvement of the left temporal region, periauricular region and infratemporal fossa with lytic destruction of left middle ear ossicles, mastoid and squamous part of temporal bone with intracranial extension. The enormity of the present lesion along with its bony erosions raised the strong clinical suspicion of malignancy. The underlying case report highlights the relevance of exhaustive sectioning and immunohistochemistry to reach the diagnosis.
Aggressive, Keratinisation, Recurrence
Case Report
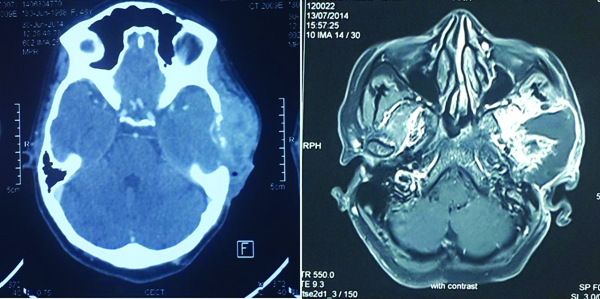
A 45-year-old woman presented to the Ear, Nose and Throat (ENT) out patient department with pain and swelling in the left ear for the past eight months. The swelling gradually increased in size to involve the entire temporal region. She also had foul smelling discharge from the left ear. She gave a past history of surgery in the left ear at the age of six years, the records of which were not available. She has had loss of hearing in the left ear ever since. On examination, she had preauricular ulceration, left facial nerve palsy and yellowish discharge in the left external auditory canal. Fistula test was negative. All other cranial nerves were normal. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) was done which showed a large heterogeneous lesion measuring 7x6.5x5.1 cm, involving the left temporal region, periauricular region and infratemporal fossa with lytic destruction of left middle ear ossicles, mastoid and squamous part of temporal bone with intracranial extension. However, parenchymal infiltration was not seen [Table/Fig-1].
CT and MRI images showing a large heterogeneous lesion measuring 7x6.5x5.1 cm, causing lytic destruction of left middle ear ossicles, mastoid and squamous part of temporal bone with intracranial extension.
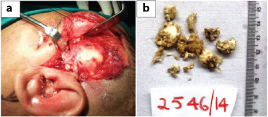
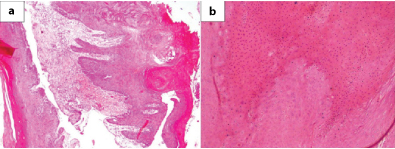
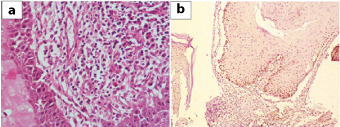
Surgical exploration was done and part of the lesion was removed which on histopathological examination showed multiple tissue fragments lined by markedly keratinizing stratified squamous epithelium with parakeratosis [Table/Fig-2,3]. Underlying subepithelium showed congested blood vessels, minimal fibrosis and chronic inflammatory infiltrate. Hypergranulosis and koilocytic change suggestive of HPV infection was also seen. Desquamated keratin flakes were also noted. A histological diagnosis of giant aggressive cholesteatoma along with Human Papilloma Virus (HPV) induced changes was suggested considering the size and invasive nature of the lesion. Repeat surgical exploration was done to remove the entire lesion. Histopathological examination was similar to earlier findings with no malignant change, confirmed by low p53 [Table/Fig-4]. The patient was kept on follow-up and six months later was referred to higher centre for further management and was re-operated showing similar histopathology. No lymphadenopathy was detected at any point of time. She subsequently went to her native village and died two months later as was informed by her relatives, cause of which could not be ascertained.
a) Intraoperative image of the lesion; b) Friable soft tissue bits with a pearly-white surface.
a) Microphotograph showing strips of markedly keratinized squamous epithelium. (H&E, ×40); b) Higher magnification showing marked parakeratosis and focal koilocytic change (H&E, x100).
a) Microphotograph showing focal subepithelial lymphoplasmacytic infiltrate (H&E, ×400). b) Microphotograph showing only basal expression of p53.
Discussion
Cholesteatoma, first described by Joseph-Guichard Duverney, is a well demarcated, non-neoplastic, temporal bone lesion often described as skin in the wrong place. It is considered a misnomer and keratoma and epidermoid cyst are other possibly more accurate names suggested to describe the same [1,2].
Cholesteatomas can be classified as congenital or acquired. Congenital cholesteatomas are associated with external auditory canal atresia and first branchial arch remnants. Various theories have been postulated for the development of acquired cholesteatomas such as immigration, invagination, squamous metaplasia, basal hyperplasia, and postsurgery/post-traumatic theories. A comprehensive theory has been proposed to explain the observed characteristics of acquired cholesteatomas which suggests mucosal coupling with traction generated by interaction of migrating opposing surfaces to be the pathogenetic mechanism [1,3,4].
History of childhood surgery was elicited in the present case, which supports the post-surgery theory of acquired cholesteatomas resulting from iatrogenic implantation of epidermal elements in the middle ear [1].
Ear discharge, hearing loss, vertigo, tinnitus, otalgia and facial nerve paralysis are seen in patients with acquired cholesteatoma in decreasing order of occurrence. Facial nerve paralysis is more commonly seen with giant aggressive lesions [1]. This case presented with chronic ear discharge, hearing loss and facial nerve palsy suggesting aggressive nature of cholesteatoma.
When original disease is not completely removed from the tubotympanic cleft, it leads to development of residual cholesteatomas. Recurrent cholesteatoma however is development of new cholesteatoma, taking the form of a retraction pocket similar to the original lesion [2].
Geven LI et al., described five different cases of giant recurrent or residual cholesteatomas with duration varying from 2-50 years after first otologic surgery [5]. This patient had her first surgery at the age of six years and presented with a giant cholesteatoma 39 years later but with subsequent fulminant course.
It is very difficult to differentiate between recurrent and residual cholesteatomas. Hence, recidivism rate (recurrence and residual rates) is a variable used to evaluate risk or recidivism [2,3,5].
Cholesteatomas present as an expansile soft tissue lesion with retracted tympanic membrane blunted scutum and eroded tympanic tegmen and ossicles on CT scan and high signal intensity on Diffusion Weighted Imaging (DWI) on MRI [1]. This case on MRI showed a heterogeneous lesion causing lytic destruction of left middle ear ossicles, mastoid and squamous part of temporal bone with intracranial extension.
Cholesteatoma on histopathological examination shows stratified squamous epithelium surrounded by granulation tissue forming the perimatrix of the lesion. The perimatrix shows predominant granulation tissue and neovascularisation and the matrix-perimatrix junction is the focus of osteolytic activity. Histologically, abundant keratin lamellae are seen in the cyst cavity lined by stratified squamous epithelium in cholesteatoma. Adult cholesteatomas also demonstrate a higher degree of fibrosis; however, a cellular inflammatory infiltrate comprising of lymphocytes, plasma cells and histiocytes are seen in both adult and paediatric cholesteatomas. Cellular dysplasia is minimal to absent suggesting that cellular dysregulation isn’t a critical event in genesis of cholesteatoma [1,6]. Aggressively growing, bone destructive areas of cholesteatoma, in addition to above features, also present with papillomatous growth and koilocytic change as seen in this case. So far, size of cholesteatoma in the present case is larger than the reported cases [7].
Squamous cell carcinomas have been reported in association with both primary and secondary acquired long standing cholesteatomas. It is not yet known whether cholesteatoma is a trigger for squamous cell carcinoma or just a coexistent bystander [8,9]. This case presented with a long standing history and bone erosion and destruction raising clinical suspicion of malignancy. However, repeated extensive histopathological examination after each surgery was suggestive of cholesteatoma.
Increased cell proliferation in cholesteatomas is seen associated with upregulation of p53 [10]. Our case, despite its giant size and recidivism, did not show significant p53 expression.
HPV 6, 11 expression in cholesteatomas assessed on Polymerase Chain Reaction (PCR) varies from 3.1% to 27.3% in different studies. HPV also has been suggested as a possible aetiological factor in aggressively growing cholesteatomas. Koilocytic change and papillomatous growth suggest a possible viral aetiology on histology which can be confirmed on PCR [11–13].
A histological diagnosis of giant aggressive cholesteatoma along with HPV induced changes was suggested considering the size and invasive nature of the lesion.
Medical management of cholesteatoma is limited to controlling preoperative infections and postoperative complications. Empirical antibiotics are given to control acute infection to reduce inflammation and formation of granulation tissue even prior to culture reports. Surgical management is the mainstay of treatment of cholesteatomas, the goal being creating a dry, recurrence free ear and maintaining its functionality.
Giant cholesteatomas are known to exist for years without causing major symptoms, before they reach a significant size causing bone destruction and intracranial extension leading to life threatening complications [14]. Our case had a similar long standing history which ultimately had a fatal outcome.
Conclusion
The size and recidivity of the lesion is a source of dilemma for the clinicians regarding management and is also a pathologist’s nightmare in the given setting. In such cases, one needs to rule out well differentiated squamous cell carcinoma, requiring exhaustive multiple sections and immunohistochemistry.
[1]. Barath K, Huber AM, Stampfli P, Varga Z, Kollias S, Neuroradiology of cholesteatomasAm J Neuroradiol 2011 32:221-29. [Google Scholar]
[2]. Robinson JM, Cholesteatoma: skin in the wrong placeJ R Soc Med 1997 90:93-96. [Google Scholar]
[3]. Kuo CL, Shiao AS, Yung M, Sakagami M, Sudhoff H, Wang CH, Updates and knowledge gaps in cholesteatoma researchBio Med Research International 2015 2015:854024 [Google Scholar]
[4]. Jackler RK, Santa Maria PL, Varsak YK, Nguyen A, Blevins NH, A new theory on the pathogenesis of acquired cholesteatoma: Mucosal tractionLaryngoscope 2015 125(Suppl 4):S1-S14. [Google Scholar]
[5]. Geven LI, Mulder JJ, Graamans K, Giant cholesteatoma: recommendations for follow-upSkull Base 2008 18:353-59. [Google Scholar]
[6]. Bassiouny M, Badour N, Omran A, Osama H, Histopathological and immunohistochemical characteristics of acquired cholesteatoma in children and adultsEgyptian Journal of Ear, Nose, Throat and Allied Sciences 2012 13:07-12. [Google Scholar]
[7]. Lannella G, Savastano E, Pasquariello B, Re M, Magliulo G, Giant petrous bone cholesteatoma: combined microscopic surgery and an adjuvant endoscopic approachJ NeurolSurg Rep 2016 77:e46-e49. [Google Scholar]
[8]. Takahashi K, Yamamoto Y, Sato K, Sato Y, Takahashi S, Middle ear carcinoma originating from a primary acquired cholesteatoma: a case reportOtol Neurotol 2005 26:1058 [Google Scholar]
[9]. Rothschild S, Ciernik IF, Hartmann M, Schuknecht B, Lütolf UM, Huber AM, Cholesteatoma triggering squamous cell carcinoma: case report and literature review of a rare tumorAm J Otolaryngol 2009 30:256-60. [Google Scholar]
[10]. Huisman M, Cholesteatoma epithelium is characterized by increased expression of Ki-67, p53 and p21, with minimal apoptosisActaoto-Laryngologica 2003 123:377-82. [Google Scholar]
[11]. Stremlau A, Helms J, Müller-Hermelink HK, Hoppe F, de Villiers EM, Detection of DNA of human papillomaviruses (HPV) in an “aggressively” growing cholesteatoma. Is cholesteatoma a virus-induced tumor?HNO 1995 43:03-05. [Google Scholar]
[12]. Chao WY, Chang SJ, Jin YT, Detection of human papillomavirus in cholesteatomasEur Arch Otorhinolaryngol 2000 257:1203 [Google Scholar]
[13]. Bai Y, Yan L, Li S, Bai Q, Expression of human papillomavirus DNA in cholesteatoma of the middle earZhonghua Er Bi Yan HouKeZaZhi 2000 35:352-55. [Google Scholar]
[14]. Shihada R, Brodsky A, Luntz M, Giant cholesteatoma of the temporal boneIsrael Medical Association Journal 2006 8:718-19. [Google Scholar]