Breast carcinoma is the most common malignant tumour and the leading cause of cancer deaths in women. In India the incidence of breast cancer is rapidly rising, amounting to 25%-33% of all cancers in women [1]. Breast carcinomas are a heterogeneous group of tumours with diverse behaviour, outcome and response to therapeutic agents [2]. Prognostic significance of breast carcinoma is no longer based on morphology alone. Modern day approach to cancer treatment involves identification of specific biomarkers on the tumour cells against which targeted therapy can be directed. ER, PR and Her2/neu markers are routinely evaluated in breast carcinoma. ER positive tumours are treated with anti-estrogen therapy (tamoxifan) and Her2 monoclonal antibody (trastuzumab) is effective in Her2 positive cases [3]. These markers are routinely evaluated by Immunohistochemistry (IHC) on whole sections.
While traditional techniques require processing and staining of several slides manually, TMA allows study of several cases by staining a single master slide. It saves time and cost of reagent used. It has the added advantage that all sections are processed at one time using identical conditions [4]. One of the major concerns regarding TMA is the small core size of tumour tissue and whether it is representative of the whole tumour. This study was hence conducted to determine if IHC results on TMA sections were comparable to standard whole sections.
Materials and Methods
This prospective study was conducted from January 2013 to December 2014 on 53 cases of breast carcinoma received in the Department of Pathology, Kempegowda Institute of Medical Sciences, Bengaluru, Karnataka, India. Demographic data was collected from the case files. The specimens were received in 10% formalin and fixed for 12-24 hours. Ethical clearance was obtained from the institute.
Routine bits were given along with a separate bit for IHC and TMA studies. Sections stained with H&E were studied for histopathological type, grade of tumour and lymph node involvement.
Immunohistochemistry: Almost 5 μm sections were cut on polylysine coated slides. Antigen retrieval for ER and PR was done using pressure cooker method in EDTA buffer (pH 9). Heat method was used to retrieve Her2/neu antigen using citrate buffer (pH 6). Sections were then soaked in peroxide block (1%) for 10 minutes. Monoclonal antibodies of ER, PR and Her2/neu {ER alpha, Clone EP1 (Monoclonal, DAKO), PR, Clone PgR 636 (Monoclonal, DAKO) and Her2/neu antibody (Polyclonal, DAKO)} were applied for 30 minutes followed by secondary antibody (HRPO IHC detection system- DAKO) for 30 minutes in a humidified chamber. Sections were then treated with 3,3’-Diaminobenzidine (DAB) chromogen for 10 minute. The sections were washed in distilled water and counter-stained with Harri’s haematoxylin, dehydrated using alcohol, cleared and mounted. ER and PR scoring was done using quick scoring and Her2/neu scoring was done as per guidelines of CAP/ASCO [5,6].
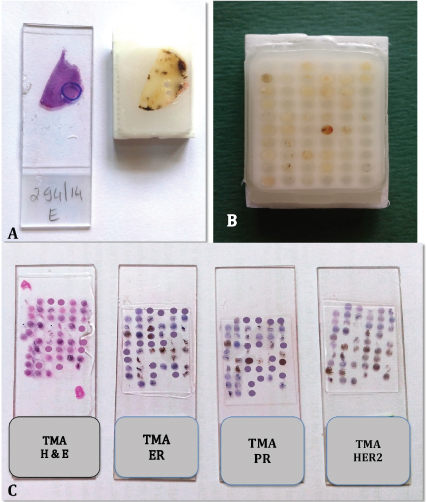
Construction of TMA: H&E sections were assessed and an appropriate area of tumour was marked on the corresponding paraffin block. Care was taken not to include areas of fibrosis, adipose tissue or necrosis [Table/Fig-1a]. A grid indicating the location of each case on the microarray block was prepared. Using a tissue microarrayer (quick Ray manual tissue microarrayer- Ultima Co. Korea) with a 2 mm diameter, a core was punched from the marked area on the block and transferred to premade recipient paraffin block (UB06-2, 6X10=60 cores). After all the sample cores were embedded, the recipient block (TMA block) was kept in the incubator at 60 degree for an hour to allow the samples to merge into the block [Table/Fig-1b]. Sections were cut and taken on regular slides for H&E staining and on polylysine slides for IHC staining. The TMA sections were stained for ER, PR and Her2/neu using the same setup as used for staining whole sections [Table/Fig-1c].
a) Marked slide and block. b) Completed tissue microarray block; c) Slides of H&E, ER, PR and Her2/neu.
Statistical Analysis
The statistical software namely SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc9.0.1, Systat 12.0 and R environment version.2.11.1 were used for the analysis of the data. Chi-square/ Fisher-Exact test has been used to find the significance of study parameters on categorical scale between two or more groups. Kappa co-efficient, Jaccard Index and G-Index were computed.
Results
A total of the 53 cases studied showed that the age ranged from 28-80 years and the mean age±SD was 52.30±12.94 years. Most cases (26.4%) belonged to age group of 41-60 years. Modified Radical Mastectomy (MRM) was done in 49 cases (92.5%) and wide excision was done in four cases (7.5%). The tumour size varied from 1.5 cm to 9 cm. In most of the cases (73.6%) the size was 2-5 cm.
The predominant histological subtype of carcinoma was IDC-NST {49 cases (92.4%)}. There was one case each of mucinous, apocrine, medullary and metaplastic carcinoma. IDC-NST was graded by using Modified Bloom Richardson’s grading. Grade II tumours (49%) predominated closely followed by Grade III tumours (44.9%).
Lymph nodes were available for study in 49 cases out of 53 as radical mastectomies were done in these cases. Total of 27 out of the 49 cases (55.1%) showed tumour deposits [Table/Fig-2].
Histopathological parameters.
| Parameters |
|---|
| Age in years | No. of cases (n=53) | % |
| <30 | 2 | 3.8 |
| 31-40 | 11 | 20.8 |
| 41-50 | 13 | 24.5 |
| 51-60 | 14 | 26.4 |
| 61-70 | 8 | 15.1 |
| 71-80 | 5 | 9.4 |
| Size in cm | No. of cases (n=53) | % |
| <2.0 | 2 | 3.8 |
| 2.0-5.0 | 39 | 73.6 |
| >5.0 | 12 | 22.6 |
| Histopathological type | No. of cases (n=53) | % |
| Infiltrating Ductal Carcinoma No Special Type | 49 | 92.4 |
| Mucinous carcinoma | 1 | 1.9 |
| Apocrine carcinoma | 1 | 1.9 |
| Medullary carcinoma | 1 | 1.9 |
| Metaplastic carcinoma | 1 | 1.9 |
| Histopathological grade | No. of cases (n=49) | % |
| Grade I | 3 | 6.1 |
| Grade II | 24 | 49 |
| Grade III | 22 | 44.9 |
| Lymph node status | No. of cases (n=49) | % |
| Positive | 27 | 55.1 |
| Negative | 22 | 44.9 |
| IHC SUBTYPE | No. of cases (n=53) | % |
| ER/PR+HER2- | 38 | 71.70% |
| ER/PR+HER2+ | 4 | 7.50% |
| ER/PR-HER2+ | 2 | 3.80% |
| ER/PR-HER2- | 9 | 17.00% |
IHC was done for ER, PR and Her2/neu on whole sections for all 53 cases. Molecular subtyping of the tumours was done. Total of 38 out of 53 cases (71.7%) were ER/PR positive and Her2/neu negative. Nine of 53 cases (17%) were triple negative. All three markers were positive in cases 4 (7.5%) and cases 2 (3.8%) were Her2/neu positive and ER/PR negative.
Comparison of ER on whole section and TMA sections: Of 53 cores 47 were available for ER study in the TMA slide as six cores were lost during IHC staining procedure. In these 47 cases, 42 were positive for ER on whole section (89.4%) and 32 were positive on TMA sections (68.1%) [Table/Fig-3a,b].
a) TMA sections showing ER positive (x100); b) ER positivity of score 7 (x400).
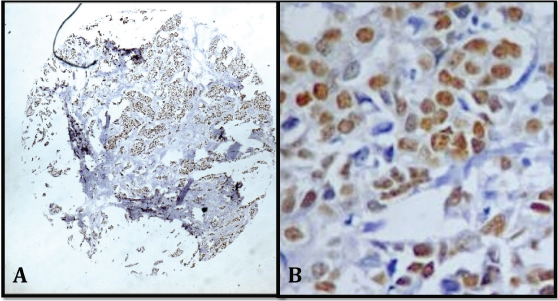
Ten out of 47 cases showed discrepancy between whole section and TMA sections. All 10 cases were positive on whole section and negative on TMA.
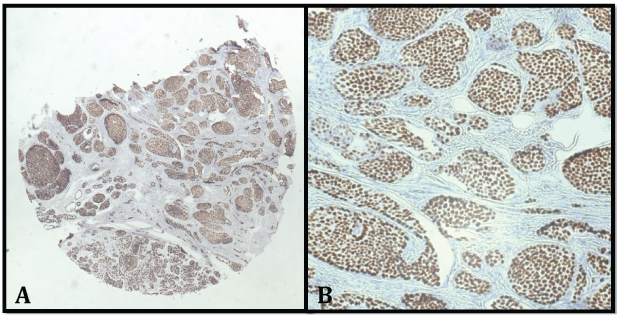
Comparison of PR on whole section and TMA sections: Of 53 cores 48 were available for PR in the TMA slide for evaluation as five cores were lost during IHC staining. Of these 48 cases, 39 were positive for PR on whole section (81.2%) and 32 were positive on TMA sections (66.7%) [Table/Fig-4a,b]. A total of 5/48 cases showed discrepancy between whole section and TMA sections. All the five cases were positive on whole section and negative on TMA.
a) TMA sections showing PR positive (x100); b) PR positivity of score 8 (x400).
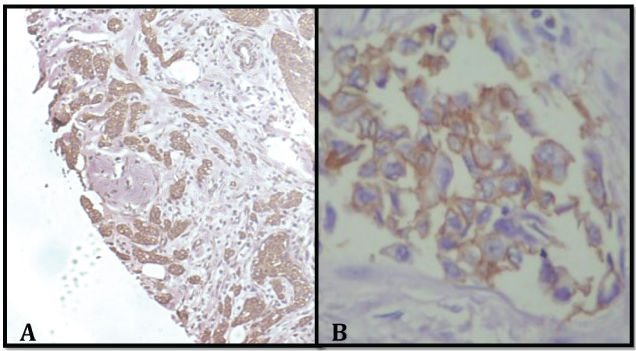
Comparison of Her2/neu on whole section and TMA sections: Total of 50 cores were available for Her2/neu in the TMA slide for evaluation as three cores were lost during IHC staining. Her2/neu was positive in six cases on both whole sections and TMA sections [Table/Fig-5a,b].
a) TMA sections showing HER2 positive 3+ (x100); b) Uniform intense membrane staining >10% of tumour cells (x400).
Comparison of TMA and whole sections: Good concordance rate was observed between TMA sections and whole sections. Concordance rate was 76.2% for ER, 82.1% for PR and 100% for Her2/neu which was statistically significant (p value<0.001). Kappa value was 0.671 for ER, 0.754 for PR and 1.000 for Her2/Neu [Table/Fig-6].
Comparison of ER, PR and HER2/neu on tissue microarray and whole sections.
| ER | PR | HER2 | p-value |
|---|
| Positive cases on whole section | 42/47 | 39/48 | 6/50 | <0.001** |
| Positive cases on microarray section | 32/47 | 32/48 | 6/50 | <0.001** |
| Positive microarray vs positive whole section | 32/42 | 32/39 | 6/6 | <0.001** |
| Concordance rate | 76.20% | 82.10% | 100% | - |
| Kappa | 0.671 | 0.754 | 1 | - |
| Jaccard Index | 0.842 | 0.861 | 1 | - |
| G-Index | 0.745 | 0.792 | 1 | - |
Abbreviation: ER- Estrogen receptor, PR- Progestrone receptor
+ Suggestive significance (p value: 0.05<p<0.10)
* Moderately significant (p value:0.01<p ≤ 0.05)
** Strongly significant (p value: p≤0.01)
Discussion
Breast carcinoma is the most common cancer in women worldwide [1]. Prognosis of this disease depends on various factors such as histological type, grade, size of tumour, lymph node metastasis and hormone status of the tumour [3]. ER, PR and Her2/neu are biomarkers recommended by American Society of Clinical Oncology (ASCO) [5,6]. Immunohistochemical classification of tumours plays a key role in deciding the treatment modality. ER and PR are important predictors of response to hormone therapy while Her2/neu overexpression is associated with poor prognosis [3]. These markers are routinely evaluated by IHC on whole sections. TMA is an innovative method of analysing these markers. It allows hundreds of cases to be evaluated in shorter period of time, while providing uniform staining of the slide. In the present study, IHC analysis for 53 breast carcinoma cases could be performed using a single slide for each of the biomarkers thereby saving time and cost of reagents.
Most of the cases belonged to age group of 41-60 years similar to studies conducted by Nikhra P et al., [7]. Majority of the tumours were Grade II and Grade III tumours, in contrast to studies conducted in Western population by Rakha EA et al., which showed a greater incidence of Grade I tumour due to early detection by screening programs like mammography and better awareness about the disease [8]. Most of the IDC-NST {37/53(69.8%)} were ER/PR positive and Her2/neu negative, similar to studies conducted by Ontilo AA et al., [9]. The medullary, apocrine and metaplastic carcinomas were triple negative. IHC subtyping of the tumours showed predominance of ER/PR positive and Her2/neu negative pattern {38/53(71.7%)}. Nine out of 53 (17%) cases were triple negative, 7.5% of the cases were positive for all three markers and 3.8% were ER/PR negative and Her2/neu positive which were comparable with studies conducted by Ontilo AA and Mohammadian K et al., [9,10].
TMA is a revolutionary technique for analysing different biomarkers.
Battifora H et al., in 1986 put together a number of tissues from different organs, in the same block to create a ‘sausage’ block, and assessed the antigen/protein distribution in the tissue [11]. In 1998, Kononen J et al., and collegues invented a mechanism for examining several histologic sections at one time by creating an array in a paraffin block [12]. These TMA were assembled by extracting small cylindrical cores from standard formalin fixed paraffin embedded tissue and embedding them within a recipient paraffin block [13,14]. TMA allows large number of samples can be processed at a time, saving time and labour [15]. All samples can be processed at identical conditions and it also conserves tissue for archival studies [16,17].
One of the limitations of TMA is that it represents a fraction of the tissue and may not be representative of whole tissue. Most studies show that the discrepancy between whole section and TMA sections may be due to heterogenicity of tumour marker. Another drawback of TMA is loss of tissue cores and sampling of non representative areas. However these limitations can be overcome by taking more number of cores and taking cores from appropriate areas which contain good number of tumour cells [18,19].
The technical difficulties encountered during the procedure in this study were:
Few of the blocks cracked while punching a core and had to be re-embedded in plastic cassettes. Using good quality wax and careful punching of cores could overcome this problem.
In some of the blocks the depth of tumour was inadequate. Ideally a uniform depth of 2 mm should be present so that multiple sections can be taken. If tumour depth is not adequate then subsequent sections may not show the tumour.
Some slides showed only small area of tumour due to surrounding fibrosis and extra care had to be taken to accurately mark the appropriate area on the paraffin block to avoid non representative sampling.
Concordance between whole sections and TMA sections were evaluated. Many studies have been conducted to validate the use of TMA for analysis of various markers [20–23] [Table/Fig-7]. Bhargava R et al., created a TMA from 114 breast carcinoma cases using four cores of 0.6 mm and reported good concordance [21]. In a study conducted by Selvarajan S et al., showed considerable agreement between Her2/neu overexpression on whole sections and TMA with kappa value of 0.724 [24]. Thomson TA et al., and Torhorst J et al., also found good correlation for ER, PR and Her2/neu on whole sections and TMA sections [25,26]. Henriksen KL et al., did a semiquantitative scoring on the 55 breast carcinoma cases and noted that as many as 89.1% and 77.8% of ER and PR scores were within one score difference [22]. They also found that the best correspondence was for Her2/neu (93%). In the present study concordance between whole section and TMA sections were comparable to Alkushi A et al., and had significant kappa value of 0.671 for ER, 0.754 for PR and 1.000 for Her2/neu [23]. However, few cases showed discrepancy between TMA and whole sections. This could be attributed to the smaller size of tissue examined as compared to whole sections. Also, ER, PR and Her2/neu are known to show some tumour heterogeneity [25]. This problem could be reduced by taking more number of cores or increasing the core size.
Comparison of whole section with tissue microarray sections.
| Zhang D et al., [20] | Bhargava R et al., [21] | Henriksen KL [22] | Alkushi A [23] | Present study |
|---|
| No. of cores | 1 | 4 | 1 | 2 | 1 |
| Size of core | 0.6 mm | 0.6 mm | 2 mm | 0.6 mm | 2 mm |
| Concordance for |
| ER | 53/56 (97%) | 107/110 (97%) | 96% | 11/15 (83.3%) | 32/42 (76.1%) |
| PR | 54/56 (98%) | 97/109 (89%) | 93% | 11/15 (83.3%) | 32/39 (82%) |
| HER2/neu | 30/33 (97%) | 94/109 (86%) | 100% | 8/9 (95.8%) | 6/6 (100%) |
There is varied opinion among researchers about the adequate number of cores and the core size. Camp RL et al., studied 2-10 microarray cores of 0.6 mm in 38 cases [16]. He concluded that analysis of two cores of TMA is comparable to whole section in more than 95% cases. Henriksen KL et al., also used single 2 mm cores similar to our study and found good concordance between whole sections and microarray sections [22]. Zhang D et al., found good concordance even on using single 0.6 mm core in his study [20]. In our study although a single core was used, the larger core size i.e., 2 mm size had adequate representative tumour and thus had significant kappa value. Two TMA slides were stained for each marker so that even if few cores floated on the first section, they could be retained on the second slide. This reduced the number of cores lost. We found that 6 (11.3%), 5(9.4%) and 3 (5.7%) cores out of the 53 cores for ER, PR and Her2/neu respectively had floated. Thomson TA et al., had similar difficulties with a core loss of 1.3% which was less when compared to Sebestian V et al., who had a higher rate of missing cores corresponding to 9% for ER, 24% for PR and 24% for Her2/neu [25,27].
Limitation
Although there are a few concerns regarding the tumour representativeness and heterogeneity of few markers, they can be overcome by taking larger cores or more number of cores.
Conclusion
In this study, a good concordance with significant kappa value was noted for ER, PR and HER2 done on whole sections and TMA sections. Thus TMA is a reliable and effective method for evaluating these tumour biomarkers. With large number of new biomarkers being discovered, it is crucial to analyse these markers on large number of cases in limited period of time. Also, it is necessary to conserve these tissue resources for further studies. Thus TMA plays an important role in the field of research can help in identifying new diagnostic and prognostic markers.
Abbreviation: ER- Estrogen receptor, PR- Progestrone receptor
+ Suggestive significance (p value: 0.05<p<0.10)
* Moderately significant (p value:0.01<p ≤ 0.05)
** Strongly significant (p value: p≤0.01)