APML is a distinct subtype (M3) of Acute Myelogenous Leukaemia (AML) with specific clinical, morphologic, and genetic features which is treated differently other than AML subtypes. The outcome of APML has been markedly improved by combination of All-Trans Retinoic Acid (ATRA) and anthracycline-based chemotherapy with maintenance treatment, but relapse still occurs in 10%–25% of the patients [1]. Occurrence of extramedullary involvement at presentation or relapse is considered as a rare event in APML [1,2]. If at all relapse occurs, the common sites involved are skin, testes, mediastinum, gingiva, ear (external auditory canal and middle ear), and CNS. Isolated CNS relapses after complete morphological and molecular remission is even rarer particularly in children (0.92% vs. 5% in adults) and very few cases have been reported in the literature till date [3,4]. Previously, the total incidence of CNS relapse in children has been found to be around 1.39%, with only 0.92% good risk patients {diagnostic Total Leucocyte Count (TLC)<10x109/L} have truly isolated CNS relapse [3]. As extramedullary relapse is rare in APML, the outcome of affected patients remains undetermined. However, overall survival for patients after an isolated CNS relapse is probably similar to patients after a Bone Marrow (BM) relapse [5,6]. This was the basis for doing this literature review to find the actual figure for isolated CNS relapse, so that some guidance can be given regarding treatment of children with APML.
Materials and Methods
Type of studies: We included all types of studies that reported about incidence or prevalence of isolated CNS relapse in children with APML.
Types of participants: Children upto the age of 18 years with APML diagnosed by clinical and laboratory methods (including molecular) having isolated CNS relapse were included. The relapse may be either single or multiple, and may be confirmed by either imaging modality or molecular methods or both. Those with relapse at other sites were excluded.
Search methodology and identification of studies [Table/Fig-1]: Medline (1980 to May 2016) via Ovid, Pubmed (1980 to May 2016), and Google Scholar (till May 2016) were searched systematically. Following search terms were used: (“central nervous system” (MeSH Terms) OR (“central”{All Fields} AND “nervous” {All Fields} AND “system”{All Fields}) OR (“central nervous system”{All Fields}) AND (“recurrence”{MeSH Terms} OR “recurrence”{All Fields} OR “relapse”{All Fields}) AND (“acute promyelocytic leukaemia”{All Fields} OR “leukaemia, promyelocytic, acute”{MeSH Terms} OR “leukaemia”{All Fields} AND “promyelocytic”{All Fields} AND “acute”{All Fields}) OR “acute promyelocytic leukaemia”{All Fields} OR (“acute”{All Fields} AND “promyelocytic”{All Fields} AND “leukaemia”{All Fields}). The studies were also identified by hand searching of selected journals.
PRISMA flow diagram for study.
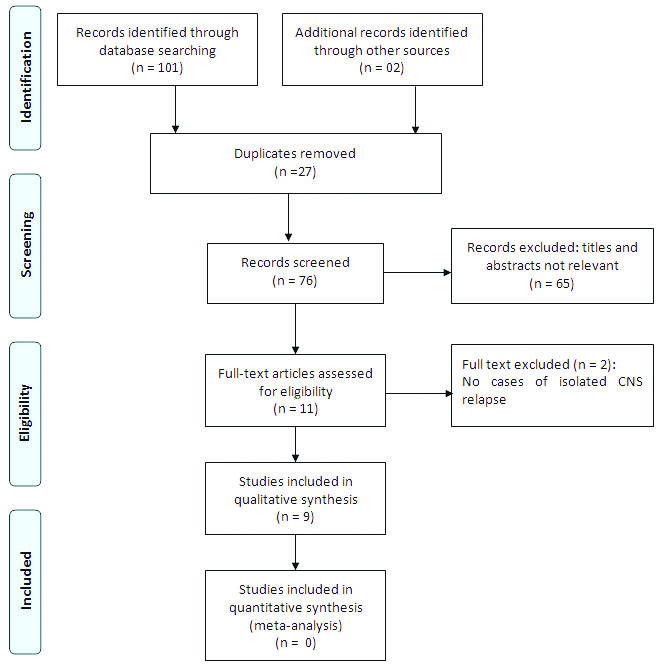
Data extraction: Data extraction form was generated and following data from the studies were extracted: author, year, country, study design, and participants (age, sex, clinical presentation, diagnosis, inclusion and exclusion criteria, treatment given, and outcome).
Results
Out of the 103 citations, nine studies were found to be eligible for inclusion as they fulfilled the criteria mentioned above [1,7–14]. Except for one study [14], all others were conducted in developed country setting. The study characteristics of all the nine studies have been described in [Table/Fig-2]. A total of 10 cases of isolated CNS relapse were reported from these nine studies. Of these 10 cases, six were male and four were female with age ranging from 3.5 years to 18 years. Majority were high risk patients except in two patients in whom the risk factors were not available in the case study [9,10] and in another described by Mittal R et al., [13]. Around 60% children were ≤six-year-old. Around 70% were having high-risk score. Nearly 50% of the cases were found to have the mean time to relapse being <one-year and most (60%) were male. Six were reported to be alive (for periods varying from 18 to 60 months), of them three being male and three female (equal survival rate). Mortality was reported across all treatment groups. The children who died were having shorter time to CNS relapse (around 12 months), were older children (>6 to 18 years). In one study, one patient relapsed twice, but was salvageable [8]. High-score was not necessarily present in children who died, but they have molecular marker that was positive by PCR.
Cases of isolated CNS relapse in paediatric APML [1,7–14].
| Author | Age (yr)/Sex | Risk Score* | Time to CNS relapse | Presentation | BM for PML-RARA(PCR) | Treatment | Follow up |
|---|
| de Botton S et al., [1] | 16/M | High | 6 months | Meningeal signs and symptoms | PML-RARA positive | ATRA + ITT+ AraC+ Anthracycline (mitoxantrone/idarubicine) | Died, seven months |
| de Botton S et al., [1] | 6/F | High | 49 months | Meningeal signs and symptoms | PML-RARA positive | ATRA + ITT+ AraC+ Anthracycline (mitoxantrone/idarubicine) | Alive, 54 months |
| Nagai S et al., [7] | 18/M | High | 2 months | Headache | Post-BMT.PML-RARA positive | Gemtuzumab ozogamicin | Alive, duration not mentioned |
| Classen CF et al., [8] | 3.5/M | - | 13 months | Asymptomatic | PML-RARA negative | Cranial irradiation(18 Gy)+IL2 | Alive, two years nine months after second BMT |
| | 23 months | General weakness and repeated focal fits | PML-RARA negative | German AML relapse study protocol+ ATRA+ ITT + allogenic BMT |
| Wang H et al., [9] | 6/F | | Not mentioned | Not mentioned | PML-RARA positive | Arsenic trioxide+ATRA | Died, 15 months after reinduction |
| Scheinemann K et al., [10] | 13/M | High | 7 months | Ataxic gait, tremors, and numbness of both legs | PML-RARA negative | ITT+ intravenous high-dose Ara-C+ autologous stem cell transplantation | Alive, 18 months |
| Montesinos P et al., [11] | 6/F | High | 49 months | NA | PML-RARA negative | ITT (x8)+ RT (18 Gy)LPA99 protocol | Alive, 59 months |
| Johnston DL et al., [12] | 6/M | High | 6 months | Left sided weakness/numbness | BM morphology remission, but PML-RARA positive on PCR | ITT +Arsenic and ATRA + Cytarabine + stem cell transplant. | NA |
| Mittal R et al., [13] | 18/M | Low except dim CD56 | 14 months | Weakness in left upper and lower limbs | PML-RARA negative | ITT(weekly)+ high dose Cytarabine | Died, 10 days after initiation of salvage chemotherapy |
| Ortega JJ et al., [14] | 6/F | High | 32 months | NA | PML-RARA negative | Mitoxantrone+high dose Ara C+IT chemotherapy | Alive |
M: Male; F: Female; ITT: Intrthecal Triple Therapy; ATRA:All-Trans Retinoic Acid; RT: Radiotherapy; BMT: Bone Marrow Transplantation; NA: Not available; PML-RARA: Promyelocytic Leukaemia/Retinoic Acid Receptor Alpha
*Low-risk patients have TLC count ≤10x109/L and a platelet count ≥ 40 x109/L; High-risk patients have TLC count ≥10x109/L.
Discussion
Incidence: In a review by Chow J et al., analyzing 81 reports of paediatric patients with APML till the year 2007, relapses were mentioned in only six cases. Of these, only one had combined BM relapse, whilst in another, the BM status was unknown [3]. In this updated review till May 2016, we could find only 10 cases of isolated CNS relapse. This shows the fact that the incidence of isolated CNS relapse in APML is very rare. As described in [Table/Fig-2], time to isolated CNS relapse in children may be from end of consolidation to 49 months.
Clinical features: The patients with CNS relapse may be asymptomatic or present with clinical manifestations depend on the size of leukemic infiltration, the sites and number of sites involved. Patients with CNS involvement present with headache, altered mental status, gait disturbances, vomiting, loss of consciousness, seizures, sensory abnormalities, and papilloedema [1,5,7–14]. There may be associated diplopia, hearing and visual loss, facial numbness or dysphagia. Spinal involvement results in focal weakness of upper or lower limb, paresthesia, back pain, radicular pain, bladder, and bowel dysfunction. The clinical features described in the studies included in the present review are in accordance with these features [1,5,7–14].
Risk factors: Risk factors for the development of CNS relapse in APML described in various studies are: TLC ≥10x109/L, advanced age, atypical morphology, presence of FLT3-ITD mutation, presence of bcr3 PML-RARα breakpoint fusion oncogene, Disseminated Intravascular Coagulation (DIC) at presentation, a very high Lactate Dehydrogenase Enzyme (LDH) level at diagnosis, and the occurrence of CNS hemorrhage before or during remission induction [15].
Pinkel D and Woo S postulated that infants and young children are more susceptible than adolescents and adults to develop CNS leukaemia because of a relatively higher vascularity in the leptomeninges [16]. In the present review, we found that 60% children were ≤six-year-old. As found previously, children with APML having high-risk score are more prone for CNS relapse is also supported by our finding, as 70% were having high-risk score. Nearly, 50% of the cases were found to have the mean time to relapse being <one-year and 60% of total cases were male. Mittal R et al., described a patient who was low-risk APML based on TLC and platelet count, but had dim CD56 positivity, which is a poor prognostic factor [13]. CD56 is a sialoglycoprotein corresponding to the neural cell adhesion molecule. It is encoded by a single gene on chromosome 11 (11q23) and it is normally expressed in a number of tissues including the brain. Previous studies have shown that CD56 expression in ALL maybe associated with an increased risk of CNS disease. Studies have shown CD56 to be an independent risk factor, being associated with both BM relapse post-remission and extramedullary relapse, irrespective of the presence or absence of other risk factors [14,17,18].
Radiology: Computed Tomography (CT) and gadolinium-enhanced brain and spine Magnetic Resonance Imaging (MRI) are used to evaluate patients with suspected CNS involvement. MRI is superior to CT to explore CNS localization. Enhancement and/or enlargement of cranial nerves, nodular or linear leptomeningeal enhancement extending into sulci or basal cisterns, and intra-dural enhancing nodules are indicative of CNS disease. However, normal MRI imaging cannot exclude occult CNS disease. Most of the studies included in the present review either used one or all of these imaging modalities to diagnose CNS involvement [1,7,8,10–14]. Few studies also did CSF study to analyze the PML-RARA gene as a confirmatory method for CNS relapse [9].
Diagnosis: In APML with CNS involvement, CSF analysis reveals diffuse infiltration by myeloblasts and promyelocytes with intense azurophilic granules, bilobed nuclei, and dispersed chromatin. Auer rods, single as well as multiple, are present. The cellular morphology of APML although provides the most rapid means for diagnosis, its unique leukaemic cell phenotype provides further confirmatory diagnostic evidence [13,19]. Flow cytometric immunophenotypic profile in blood and BM is 100% sensitive for screening of APML irrespective of the morphologic variety and karyotype complexity. Immunophenotyping helps even when cryptic translocations are unrecognizable by cytogenetic analysis or FISH analysis for t (15;17). In flow cytometry studies, a single major cluster population spanning a wide range of side scatter on a scatter dot plot appearing as a teardrop or triangle shape suggests an APML profile. A triad of absent or weak CD34, absent HLA-DR, and consistent CD117 expression is distinctive for APML. Other common features include consistently strong CD13 and CD33, and absent or low-level expression of CD10, CD11a, CD11b, CD11c, CD18, CD45RO, CD105, and CD133. There is expression of CD9, CD68 and aberrant expression of CD2 and CD56 in a subset of APMLs with a possible poor prognosis [8]. RT-PCR confirms the presence of PML-RARA transcripts, and immunophenotyping and RT-PCR for PML-RARA is required for a definite diagnosis. All the studies included in the present review used one or most of these modalities to diagnose CNS involvement [1,7–14].
Treatment: The introduction of ATRA; tretinoin into the therapy of APML completely revolutionized the management and outcome of this disease [20]. For patients without leukocytosis, who have an extremely low risk of CNS relapse, CNS prophylaxis is not indicated [11]. In contrast, in the presence of high-risk features along with a high WBC count, CNS prophylaxis with intrathecal chemotherapy is required. The prognostic factors for CNS relapse in children with APML need to be strictly defined to start CNS prophylaxis at the time of initial diagnosis. The present review shows that a non-aggressive approach to treat APML cases is justified considering availability of very good chemotherapeutic agents and lower risk of CNS relapse.
Survival: For children with APML, survival rates exceeding 90% are now achievable by rapid initiation of ATRA combined with chemotherapy and appropriate supportive care measures. Despite this, 15%-25% cases still relapse [1,15,20]. As extramedullary relapse is rare in APML, the outcome remains largely undetermined; however one study reported the outcome being similar to that of patients who experienced isolated BM relapse [10]. Of the 10 patients, six were reported to be alive (for period varying from 18 to 60 months), of them three being male and three female (the survival rate of either being essentially same (60%). Mortality was reported across all the treatment groups. The children who died were having shorter time to CNS relapse (around 12 months), were older children (>6 to 18 years). High-score was not necessarily present in children who died, but they have molecular marker that was positive by PCR.
Strengths: The strengths of the present review are: it included a comprehensive and systematic search of literature to find eligible studies; identified the gap in knowledge that gives direction to future studies.
Limitation
There are some potential limitations that merit attention: (a) methodological issues - all the studies were though observational, were limited to case reports or series, and were conducted mostly in developed country settings countries; (b) dramatic change in treatment and prognosis in APML during the last decade; (c) we could not pool the results from the studies to calculate the cumulative prevalence of isolated CNS relapse.
Conclusion
In the present review, disease in the high-risk group, male sex, younger age (≤6 years), and PML-RARA detection was found to be associated with isolated CNS relapse in children with APML. These in addition to previously described prognostic factors should be taken into account while deciding therapy at the time of initial diagnosis of APML cases. CSF examination along with immunophenotyping and RT-PCR for PML-RARA is required for a definite diagnosis and early treatment of patients to improve overall survival.
M: Male; F: Female; ITT: Intrthecal Triple Therapy; ATRA:All-Trans Retinoic Acid; RT: Radiotherapy; BMT: Bone Marrow Transplantation; NA: Not available; PML-RARA: Promyelocytic Leukaemia/Retinoic Acid Receptor Alpha
*Low-risk patients have TLC count ≤10x109/L and a platelet count ≥ 40 x109/L; High-risk patients have TLC count ≥10x109/L.