Angiolymphoid Hyperplasia with Eosinophilia Involving the Occipital Artery: Case Report and Review of Literature
Laia Fite-Trepat1, Miriam Martos-Fernandez2, Margarita Alberola-Ferranti3, Alba De Pablo-Garcia-Cuenca4, Coro Bescosatin5
1 Resident, Department of Oral and Maxillofacial Surgery, Vall Hebron Hospital, Barcelona, Spain.
2 Resident, Department of Oral and Maxillofacial Department, Vall Hebron Hospital, Barcelona, Spain.
3 Pathologist, Department of Pathology, Vall Hebron Hospital, Barcelona, Spain.
4 Assistant Surgeon, Department of Oral and Maxillofacial Surgery, Vall d’Hebrón Hospital, Barcelona, Spain.
5 Head, Department of Oral and Maxillofacial Surgery, Vall d’Hebrón Hospital. Barcelona, Spain.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Laia Fite-Trepat, Departamento De Cirugía Oral y Maxilofacial, Planta 9 Passeig de la Vall d’Hebrón, 119 -129 08035 -Barcelona, Spain.
E-mail: laiafitetrepat@gmail.com
Angiolymphoid Hyperplasia with Eosinophilia (ALHE) is an atypical vascular tumour occurring primarily in the head and neck area, which must be distinguished from Kimura’s disease. The lesions can appear as single or multiple grouped intradermal papules or subcutaneous nodules. We report a rare case of ALHE in a 57-year-old female with a large lesion of three nodules involving the right occipital artery which had a long term evolution and we treated it by surgical excision. The definitive histopathological diagnosis was ALHE. Our case report is accompanied by a discussion of clinical, radiological and histological features. Surgical excision with free margins is the treatment of choice but, even though ALHE is considered a benign condition, recurrence is common.
Epithelioid haemangioma, Head and neck, Kimura’s disease, Occipital region, Scalp, Surgical treatment
Case Report
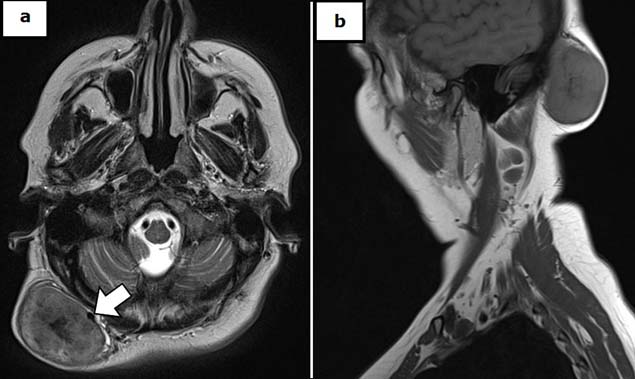
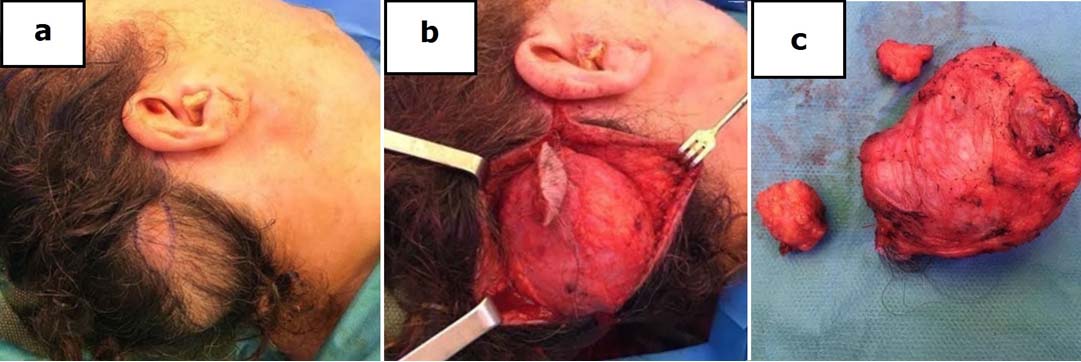
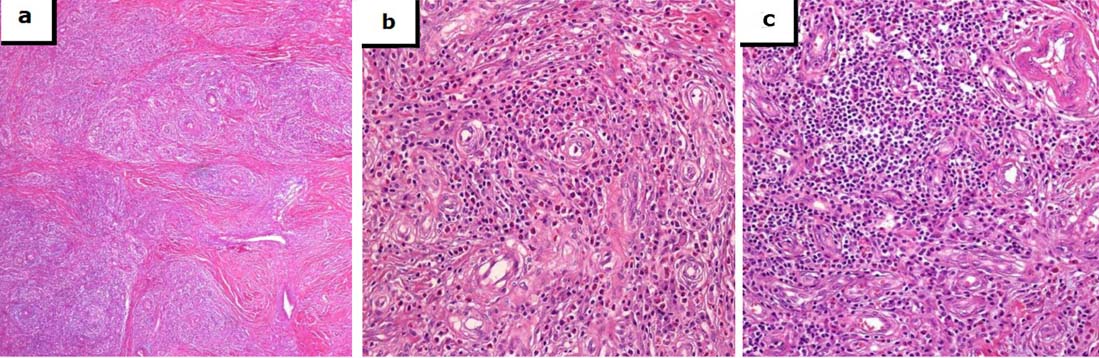
A 57-year-old Caucasian female consulted our clinic to assess the appearance of a large right occipital soft and well-defined swelling which had a progressive growth during last three years. It was not accompanied by pain, swelling, pruritus or bleeding [Table/Fig-1]. She denied any medical history of interest or trauma. On examination, she had a non-tender swelling, which was 6 cm x 6 cm in size, firm in consistency and without evidence of regional lymphadenopathy at physical examination. Initial laboratory values did not reveal peripheral eosinophilia or any other disorders. MRI showed a well-defined subcutaneous right suboccipital solid mass of 57 mm x 32 mm x 58 mm, with high cellularity and diffusion restriction, in contact with the occipital triangle muscles and the skull but without signs of bone erosion. Two smaller nodules of similar radiologic characteristics accompanied the main lesion. The biggest lesion had intense contrast enhancement, and a vascular supply through a branch of the occipital artery was observed [Table/Fig-1]. The differential diagnosis included a benign vascular lesion or neurofibromatosis, without being able to discard a malignant tumour. A surgical biopsy was performed and the histopathological result was diagnostic of Angiolymphoid Hyperplasia with Eosinophilia (ALHE). In January 2016 surgical removal of the three tumours under general anaesthesia was performed through a right occipital approach concealing the incision inside the hairline. The lesions showed a rich vascular supply and were located in a subcutaneous plane with well-defined margins without underlying muscle or bone infiltration [Table/Fig-2]. The patient was discharged two days after the surgery without any complications. The histopathological examination showed a vascular lobular proliferation of small and medium blood vessels lined by plump, epithelioid endothelial cells with a rich perivascular infiltrate of mature lymphocytes which was admixed with numerous eosinophils without germinal center formation or eosinophilic abscess formation suggestive of ALHE [Table/Fig-3]. On follow-up, after nine months post-operatively, the patient had no pain, no pruritus, a correct healing and no signs of clinical recurrence.
Contrast MRI T1 scan showing a large mass in the subcutaneous occipital plane with a vascular supply through an arterial pedicle from a branch of the occipital artery [white arrow]. a) axial view. b) sagittal view.
Intraoperative images. a) well-defined tumor of 6x6cm. b) exposure of the lesion through a right occipital approach. c) Macroscopic image of the surgical specimen (7x6x4cm) composed of three nodules.
Histopathological examination images. a) H&E: Lobular proliferation of small and medium blood vessels. (4X) b) H & E: Perivascular infiltrate with numerous eosinophils. (10X) c) H&E: Vascular proliferations lined by plump, epithelioid endothelial cells with inflammatory infiltrate (lymphocytes and eosinophils) (10X).
Discussion
ALHE is an uncommon, benign, vascular and inflammatory disorder involving the skin microvasculature with an undetermined pathogenesis. This anomaly was first described by Wells GC and Whimster IW in 1969 [1,2]. Initially, this entity and Kimura’s disease were considered part of the same disease spectrum. Nowadays it is known that ALHE has distinctive histological features and both are categorized under eosinophilic dermatoses [1,3]. The main characteristics of ALHE and Kimura’s disease are shown in [Table/Fig-4] [4].
Main characteristics of ALHE and Kimura’s disease.
| Characteristics | ALHE | Kimura’s Disease |
|---|
| Clinical Presentation |
| Age | 3rd-5th decade | 1st-3rd decade |
| Sex | Female (70%) | Male (85%) |
| Race | All | Asian |
| Site | Head and neck | Head and neck, salivary glands |
| Size of skin lesion | About 1cm | About 3cm |
| Growth progression | Longer (up to 25 years) | Shorter (1-4 years) |
| Aetiology | Unknown. Vascular neoplasm vs reaction at vascular traumatic insults | Autoimmune disorder, allergic or neoplasic reactions, insect bites, parasites, infection |
| Systemic Disease |
| Lymph node involvement | Rare (5 to 20%) | Common (50-75%) |
| Salivary gland involvement | Rare | Common |
| Renal involvement | Rare | Common (about 20%) |
| Recurrence rate | 30% | 15-40% |
| Peripheral eosinophilia | Usually not present | Usually present (98%) |
| Concentration of IgE (serum) | Normal | High |
| Pruritus | Yes | No |
| AV Shunts | Usually | Rare |
| Histological Features |
| Neoangiogenesis | Prominent | Less prominent |
| Plump, epithelioid endothelial cells | Present | Absent |
| Lymphoid follicles | Rare (10%) | Common |
| Eosinophilic abscesses | Not seen | Present |
| IgE deposits in germinal center | Absent | Present |
| Fibrosis | Absent | Present |
Regarding its epidemiology, the latest revisions indicate that there is no sex or race predominance and the mean age of presentation is the fourth decade [5,6]. Adler B et al., also described that the typical appearance is a firm single (53.4%) or multiple (46.6%) nodules in the dermis or subcutaneous tissue, which can be asymptomatic or can be accompanied by pruritus (about third of cases), pain or bleeding, with a benign and chronic clinical evolution [5]. However, it remains unclear how often symptoms occur and if they have any prognostic value, the certain is that cases reports of the literature that we have reviewed manifested minor symptoms or were affected for the mass effect at the anatomical region. Systemic eosinophilia is rare. The head and neck region is the most frequent location, with predilection to the preauricular area (36.3%), face (28.2%) and scalp (17.3%) [4]. Conversely, it’s rarely seen on the trunk, extremities [7], genitalia or in an extracutaneous location (orbit, lacrimal gland, oral mucosa, tongue, lung or intestinal mucosa) [8–10]. Injuries to the temporoparietal area are more likely to form large clusters. On the contrary, those located in other regions are usually solitary and well defined with better treatment response. Despite spontaneous involution has been described in 17 cases [5], this is exceptional, and no publication has reported malignant transformation [10]. This is an important datum because most of the patients consult for the injury months or years after its first manifestation, if ALHE was an injurious disease we would be in another context.
Aetiology could be related to hormones (hyperestrogenism, pregnancy) [6], infection (Herpes Virus 8, Human polyomavirus-6) [11], immunological factors, mild trauma (bites, vaccinations) or environmental factors (there is an increased prevalence in coastal communities) [5]. The expression of endothelial markers such as factor VII, CD34 or VEGF [1,12] has been reported. However, this is not an essential condition and its pathogenesis remains uncertain [13]. The main entities to consider for differential diagnosis are epithelioid haemangioendothelioma, extranodal lymphoma, pyogenic granuloma, insect bite (which is characterized by a more florid mixed inflammatory infiltrate without vascular proliferation), angiosarcoma, Kaposi’s sarcoma (slit-like spindle cells within vascular space with extravasation of erythrocytes and positive HHV [8], angioma (vascular proliferation without eosinophilic component) and cutaneous metastases [4].
Although therapeutic management is not well defined, there are multiple treatment options. Maybe this fact reflects the knowledge gap related to its true pathogenesis. As Adler BL et al., reflects in their systematic review, surgical excision is the most accepted treatment (44.2%), despite the difficulty in identifying the required surgical margin [5]. As we commented earlier, ALHE is not an aggressive disease and that’s why a lot of other relevant and non-invasive treatments are cited on the literature: intralesional interferon-α, oral isotretinoin (which affects the angiogenesis process at a dose of 0.5 mg/Kg/day), topical imiquimod, systemic or injected steroids, injection of cytotoxic drugs, micrographic surgery, cryotherapy, intralesional radiofrequency ablation and laser therapy, the latter having a promising future due to low recurrence rates and its ability of being a good option for elderly or non-surgical patients [5,14]. A case report describes a 53-year-old man with frontal scalp ALHE lesion, received intralesional radiofrequency ablation avoiding an epidermal damage and having a reduction about 75%-80% of the injury, after three years of follow up he hasn’t had any recurrence [14]. Recently, an interleukin-5 based therapy has been described to be effective [12,15], which can meddle in the production and activation of eosinophils. Some studies have reported the use of a daily oral dose of propranolol 40 mg in order to reduce the lesion size. It has been identified that surgical excision, dye laser and carbon-dioxide laser are the therapeutic options with less treatment failure (defined as incomplete resolution or recurrence after treatment of the disease) with 40.8%, 50% and 54.6% respectively, and the highest rates are for systemic (87.8%) and topical corticosteroids (98.2%) [5]. Spontaneous resolution is an unusual behaviour of ALHE and treatment failure has been related to early age of onset, longer duration, multiple lesions and presence of symptoms.
In our case, the patient was a healthy mid-age woman with an important mass at occipital scalp, being a suspect of ALHE which involved the occipital artery. Surgery was the therapy of choice because of the size of the lesion and its aesthetic involvement. During surgery a wide surgical margin was able to achieve. Nine months later the patient remains asymptomatic without signs of local recurrence. Despite surgical excision remains to be the most effective treatment option, its recurrence rate is closely related to the identification of clear surgical margins to excise the lesion properly. This implies the need for further research into the required surgical margin necessary to excise the lesion properly.
Conclusion
We can conclude that, may be because we don’t know the essential aetiology, and ALHE is a rare disease, multiple treatments had been tested without find a non-invasive standard treatment. It’s important to highlight that there is few published literature and the studies revised are insufficient to conclude and identify a standard treatment because current knowledge derives from retrospective reports and case series, leading to a non-evidence-based treatment approach. Although, surgery is the best option due to having the lower recurrence rate. Therefore, whatever the treatment of choice applied, it is essential to carry out close monitoring of the patient to detect possible recurrences.
[1]. Wells GC, Whimster IW, Subcutaneous angiolymphoid hyperplasia with eosinophiliaBr J Dermatol 1969 81:1-14. [Google Scholar]
[2]. San Nicoló M, Mayr D, Berghaus A, Angiolymphoid hyperplasia with eosinophilia of the external ear: case report a review of the literatureEur Arch Otorhinolaryngol 2013 270:2775-77. [Google Scholar]
[3]. Kamath MP, Bhojwani KM, Bhandarkar AM, Pai RR, Rent NH, Angiolymphoid hyperplasia with eosinophilia of root of nose: a rare phenomenonJ Clin Diagn Res 2014 8:144-45. [Google Scholar]
[4]. Ramchandani PL, Sabesan T, Hussein K, Angiolymphoid hyperplasia with eosinophilia masquerading as Kimura diseaseBr J Oral Maxillofac Surg 2005 43:249-52. [Google Scholar]
[5]. Adler BL, Krausz AE, Minuti A, Silverberg JI, Lev-Tov H, Epidemiology and treatment of angiolymphoid hyperplasia with eosinophilia (ALHE): A systematic reviewJ Am Acad Dermatol 2016 74:506-12. [Google Scholar]
[6]. Olsen TG, Helwing EB, Angiolymphoid hiperplasia with eosinophilia, a clinicopathologic study of 116 patientsJ Am Acad Dermatol 1985 12:781-96. [Google Scholar]
[7]. Ristiano G, Gupta A, Burke F, Angiolymphoid hyperplasia with eosinophilia in the hand: a case reportJ Hand Surg Eur 1990 15:376-77. [Google Scholar]
[8]. Ueda S, Goto H, Usui Y, Nagai T, Nagao T, Angiolymphoid hyperplasia with eosinophilia occurring in bilateral eyelidsBMC Opthalmology 2013 13:38-40. [Google Scholar]
[9]. Berney DM, Griffiths MP, Brown CL, Angiolymphoid hyperplasia with eosinophilia in the colon: a novel cause of rectal bleedingJ Clin Pathol 1997 50:611-13. [Google Scholar]
[10]. Sanchez-Acosta A, Moreno-Arredondo D, Rubio-Solornio RI, Rodríguez-Martínez HA, Rodríguez-Reyes AA, Angiolymphoid hyerplasia with eosinophilia of the lacrimal gland: a case reportOrbit 2009 27:195-98. [Google Scholar]
[11]. Rascován N, Monteil-Bouchard S, Grob JJ, Collet-Villette AM, Gaudy-Marqueste C, Penicaud M, Human polyomavirus-6 infecting lymph nodes of a patient with an angiolymphoid hyperplasia with eosinophilia or Kimura diseaseClin Infect Dis 2016 62:1419-21. [Google Scholar]
[12]. Aoki M, Kimura K, Kusunoki T, Tahara S, Kawanah S, Angiolymphoid hyperplasia with eosinophilia associated with anomalous dilatation of occipital artery: IL-5 and VEGF expression of lesional mast cellsArch Deratol 2002 138:982-84. [Google Scholar]
[13]. Vandy F, Izquierdo L, Liu J, Criado E, Angiolymphoid hyperplasia involving large arteriesJ Vasc Surg 2008 47:1086-84. [Google Scholar]
[14]. Singh S, Dayal M, Walia R, Arava S, Sharma R, Gupta S, Intralesional radiofrequency ablation for nodular angiolymphoid hyperplasia on forehead: a minimal invasive approachIndian J Dermatol Venereol Leprol 2014 80:419-21. [Google Scholar]
[15]. Braun-Falco M, Fisher S, Plotz SG, Angiolymphoid hyperplasia with eosinophilia treated with anti-interleukin 5 antibody (mepolizumab)Br J Dermatol 2004 151:1103-04. [Google Scholar]