Extramedullary Solitary Plasmacytoma: Demonstrating the Role of 18F-FDG PET Imaging
Archana Gautam1, Kamal Kant Sahu2, Ahsan Alamgir3, Imran Siddiqi4, Sikander Ailawadhi5
1 Division of Hematology, Department of Oncology, Mayo Clinic, Jacksonville, FL, USA.
2 Division of Hematology, Department of Oncology, Mayo Clinic, Jacksonville, FL, USA.
3 Division of Hematology, Department of Oncology, West Virginia University, Morgantown, WV, USA.
4 Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
5 Division of Hematology, Department of Oncology, Mayo Clinic, Jacksonville, FL, USA.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sikander Ailawadhi, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL, USA 32224.
E-mail: ailawadhi.sikander@mayo.edu
An Extramedullary Plasmacytoma (EMP) is characterized by a neoplastic proliferation of clonal plasma cells outside the medullary cavity. EMPs are a rare occurrence compared to other malignant plasma cell disorders and account for approximately 3-5% of plasma-cell neoplasms. Although most cases of EMP are not immediately life threatening at diagnosis, EMPs can progress to Multiple Myeloma (MM) and thus, warrant monitoring. Currently, there are no standard guidelines for when and how to monitor patients who are diagnosed with or treated for a solitary plasmacytoma. We present a case of solitary EMP who was treated adequately and definitively but developed a distinct, non-contiguous subsequent solitary EMP and was only discovered due to surveillance 18F-Fludeoxyglucose Positron Emission Tomography (18F-FDG) (PET) scan. Uniform surveillance guidelines should be developed and the potential benefits of PET and other imaging techniques as well as their cost should be considered.
Lambda restriction, Multiple myeloma, Surveillance
Case Report
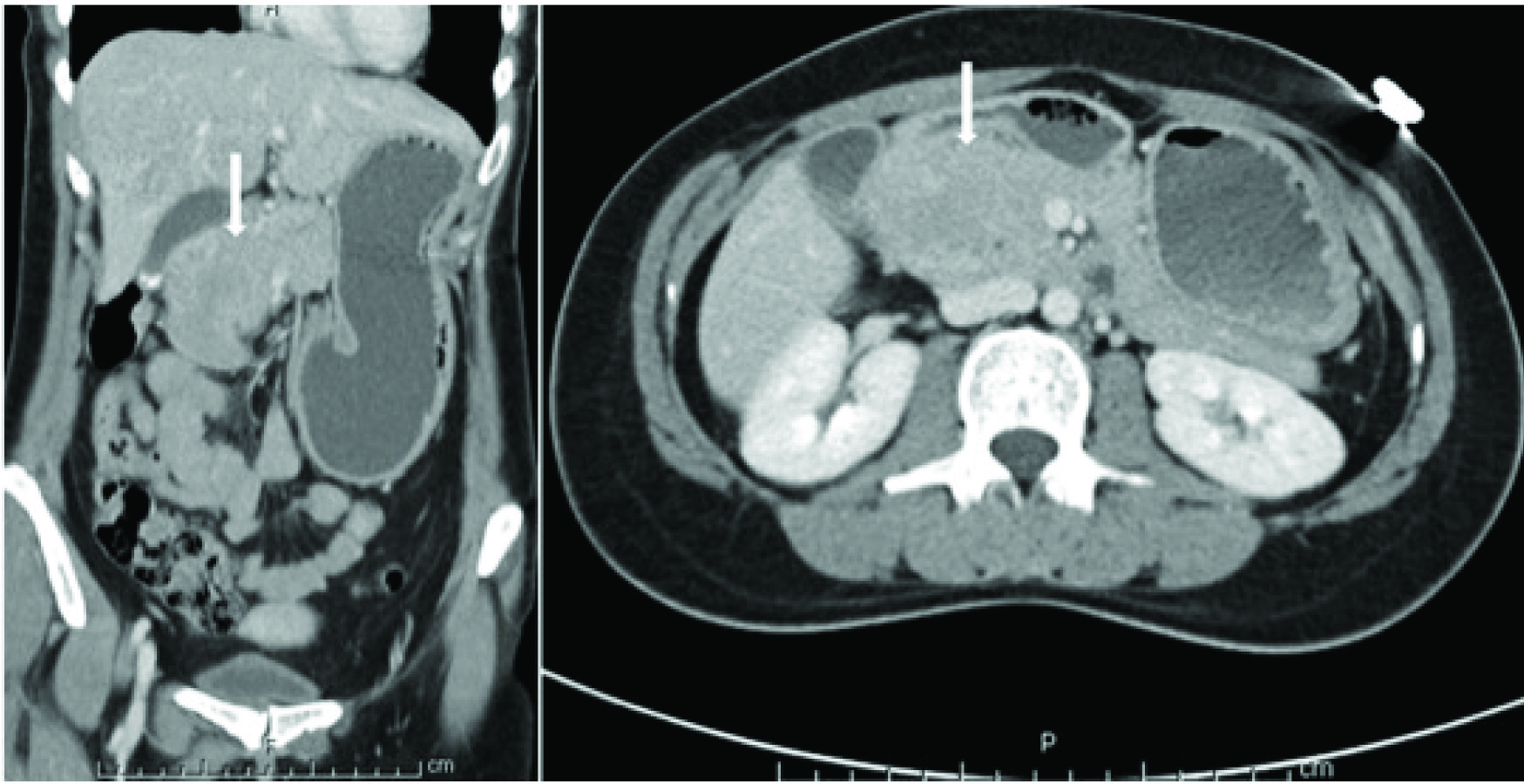
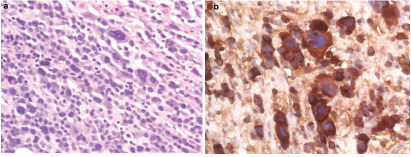
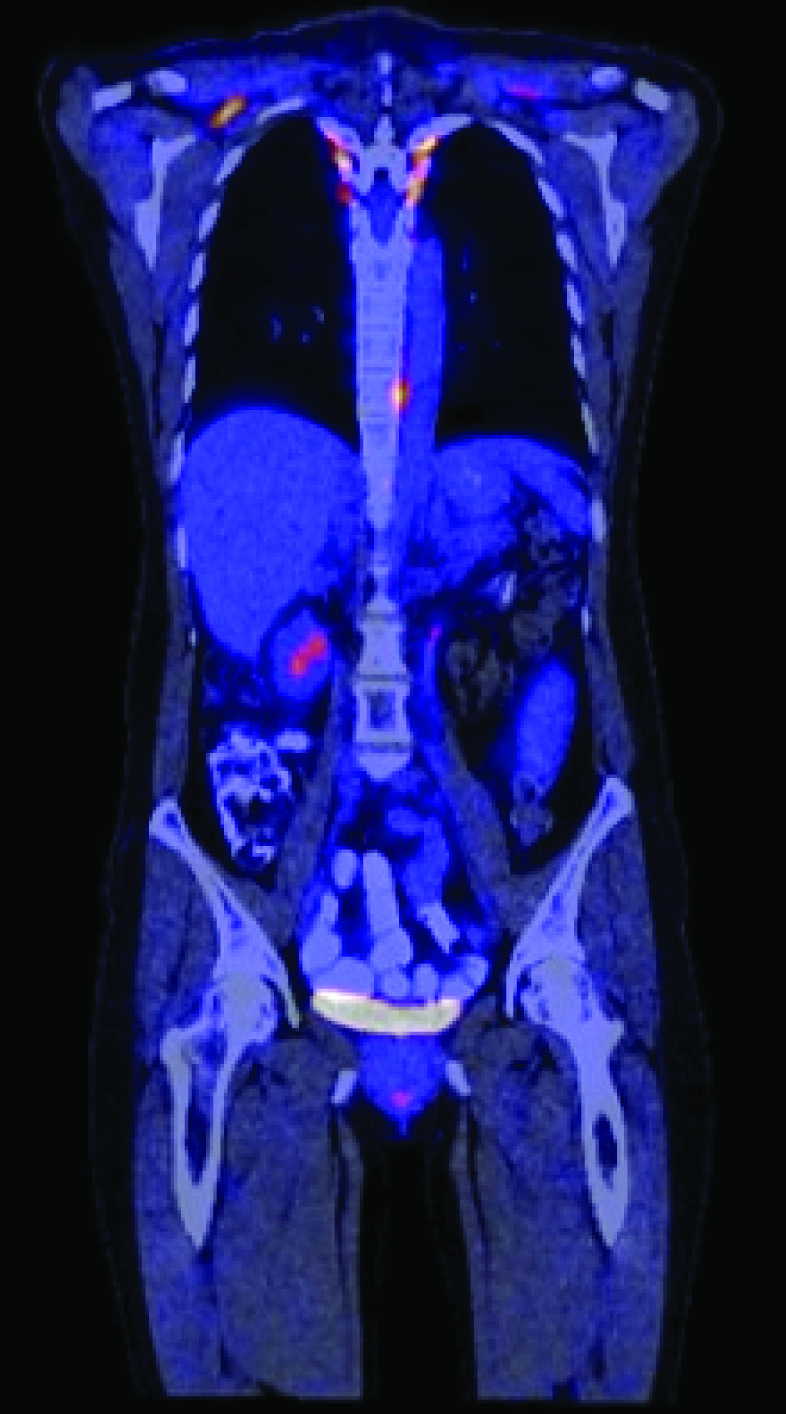
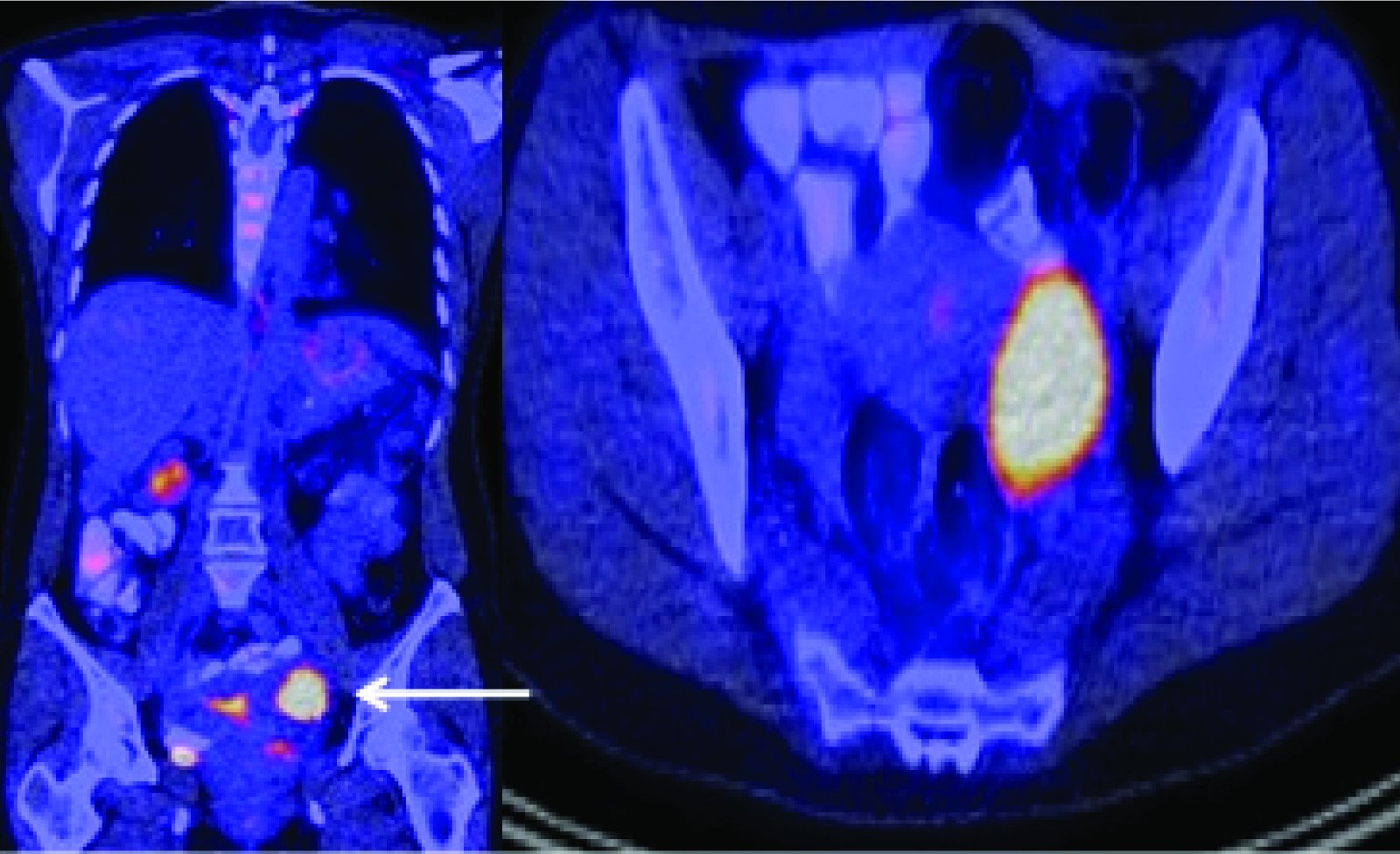
A 37-year-old female presented with significant nausea, vomiting, and abdominal pain for two weeks. On examination there were no localizing symptoms, with normal bowel sounds, diffuse mild abdominal tenderness and no organomegaly or ascites. Computed Tomographic (CT) imaging showed that she had a 5.1 cm x 4.1 cm lesion involving the second part of duodenum and the pancreatic head [Table/Fig-1]. An upper gastrointestinal endoscopy and biopsy from the lesion showed this mass was infiltrated by plasma cell [Table/Fig-2a] showing lambda-restriction [Table/Fig-2b] and were also positive for CD138 and MUM-1, while negative for kappa light chains, leading to the diagnosis of EMP. A skeletal survey did not show any abnormalities, and no clonal plasma cells were detected in the bone marrow on a core biopsy. The results of the patient’s laboratory tests were normal [Table/Fig-3]. Thus, the diagnosis of a solitary EMP was made. The patient underwent definitive involved-site radiation therapy with 45 Gy administered over a four-week period. Results from a follow up 18F-FDG PET and CT scan were normal [Table/Fig-4]. Due to persistent symptoms from adhesions, which were considered a sequela of radiation therapy, the patient underwent a subsequent whipple procedure, which resolved the symptoms. A follow up surveillance PET/CT scan was conducted three months later and was reported normal. On further routine surveillance PET/CT, approximately seven months after the primary treatment, the patient was found to have an FDG-avid, asymptomatic, left-sided adnexal mass with a maximum Standardized-Uptake Value (SUV max) of 8.7 [Table/Fig-5]. Considering the previous diagnosis of solitary EMP, the possibility of another EMP was entertained. She underwent surgical excision of the mass to confirm a diagnosis, and a histopathological evaluation confirmed it to be a plasmacytoma [Table/Fig-6]. The plasma cells were again noted to be lambda-restricted, similar to the original plasmacytoma. A repeat bone marrow biopsy and laboratory testing did not show any other abnormalities suggesting an underlying active or evolving MM. She had mild anemia, but this was microcytic, and on further workup her anemia was consistent with iron deficiency [Table/Fig-3]. Due to lack of data on the benefit of adjuvant radiation therapy in plasmacytomas having undergone definitive surgical treatment, it was not considered. She was recommended to initiate systemic therapy after the second EMP occurrence to prevent further EMP or progression to active MM but she opted against it and was followed with active surveillance. The patient received PET/CT scans every three months for the first year and then every six months thereafter. She remains free of any plasmacytoma recurrence or progression to MM three years after the second episode.
CT scan showing a duodenal mass on coronal and axial sections.
(a) Plasmacytosis in excisional biopsy specimen of duodenal/pancreatic head mass (40X) at the time of initial solitary extramedullary plasmacytoma (EMP); (b) Lambda restriction in plasma cells from excisional biopsy specimen of duodenal/pancreatic head mass (40X).
Timeline of selected laboratory parameters at the time of diagnosis and follow up.
| At the Time of GI Plasmacytoma | After Resolution of GI Plasmacytoma | At the Time of Ovarian Plasmacytoma |
|---|
| Hemoglobin | 12.1 g/dL | 12.7 g/dL | 10.6 g/dL |
| Total leucocyte count | 10.1 X109/L | 3.1 X109/L | 5.2 X109/L |
| Platelet count | 468 X109/L | 313 X109/L | 442 X109/L |
| Calcium | 9.8 mg/dL | 9.5 mg/dL | 9.2 mg/dL |
| Albumin | 4.6 g/dL | 4.6 g/dL | 4.7 g/dL |
| Creatinine | 0.6 mg/dL | 0.6 mg/dL | 0.6 mg/dL |
| Kappa light chains | 13.2 mg/L | 14.3 mg/L | 13.4 mg/L |
| Lambda light chains | 12.1 mg/L | 17.9 mg/L | 15.8 mg/L |
| Kappa/lambda ratio | 1.09 | 0.79 | 0.85 |
| Serum protein electrophoresis | Normal | Normal | Normal |
| Skeletal survey | Normal | Normal | Normal |
GI = gastrointestinal
Post-treatment18 F-FDG PET/CT scan showing resolution of hypermetabolic lesion (coronal section).
18F-FDG PET/CT scan showing a hypermetabolic soft tissue left-sided adnexal mass.
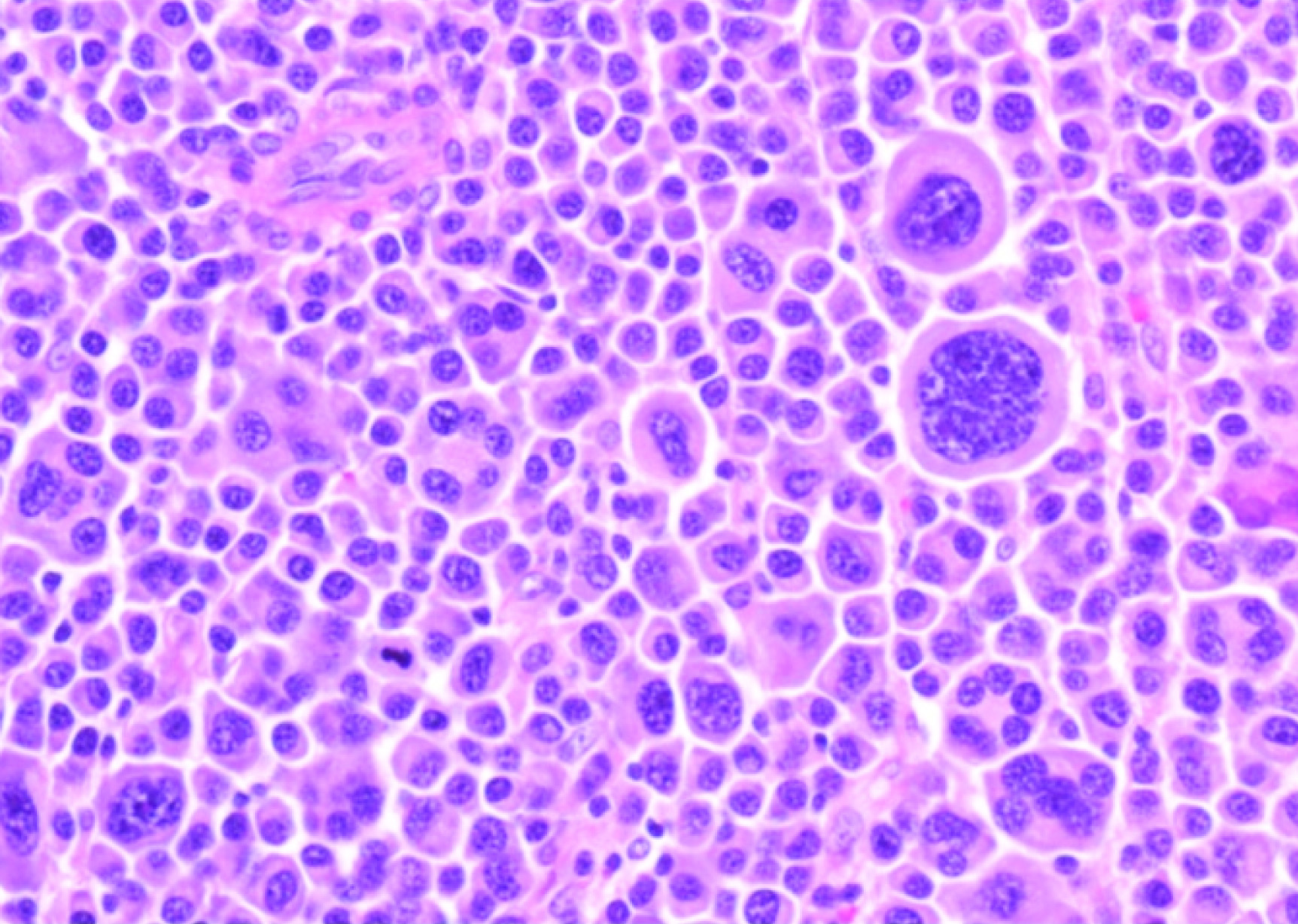
Plasmacytosis in excisional biopsy of ovarian mass (40X) at the time of recurrent EMP.
Discussion
EMPs are a rare occurrence compared to other malignant plasma cell disorders and account for approximately 3-5% of plasma-cell neoplasms [1]. The most common sites of EMP involvement are respiratory and gastrointestinal systems [2,3]. Although most cases of EMP are not immediately life threatening at diagnosis, EMPs can progress to MM and thus, warrant monitoring. Previous studies have reported that less than 7% patients with solitary bone plasmacytomas will develop a local recurrence after tumouricidal radiation [4] and approximately 10-15% of patients will ultimately develop MM with a higher (20%) progression rate in those with minimal bone marrow involvement [4,5]. Of note, less data is available regarding EMP that is not bone-based.
In recent years, the survival of patients with MM has significantly improved [6]. This is likely due to the availability of increasingly effective therapeutic agents that are well tolerated over long durations of treatment. Because of these encouraging improvements in patient outcomes and the availability of highly effective therapeutic agents, the International Myeloma Working Group (IMWG) has revised the definition of MM with an aim of preventing end-organ damage by initiating systemic treatment before such damage occurs [5]. The workup and definition of a solitary plasmacytoma as well as its risk of progression to MM has been well defined, and PET/CT scans have emerged as an important modality that helps confirm diagnoses and leads to definitive therapy in at least some cases [5]. Another imaging modality that is recommended frequently to confirm the solitary nature of an EMP is a Magnetic Resonance Imaging (MRI) of the spine and pelvis [7], but this is typically more expensive than a PET/CT scan and takes a longer duration to complete. Furthermore, while a MRI may be beneficial in detecting solitary plasmacytomas of bony origin, MRIs confined to certain body parts may miss EMP not associated with a bony lesion. The IMWG published a consensus statement regarding the use of MRI in the management of patients with MM [8]. The whole-body MRI is the recommended MR modality and its substitution by just the axial MRI can result in a significantly decreased sensitivity in recognizing lesions [9]. On the other hand a PET/CT scan can provide a faster, whole-body modality with a sensitivity of 96%, specificity of 77% and a particular advantage of detecting extramedullary disease [10]. In a meta-analysis highlighting the comparison of MRI and PET/CT, the major advantages of MRI were a higher sensitivity for detecting diffuse marrow infiltration and localized vertebral disease with increased risk of fracture, while the PET/CT detected a higher number of osteolytic lesions [11].
Despite the guidelines for diagnosis of an EMP, there are no specific published guidelines on how to perform surveillance in patients with plasmacytomas, especially using PET/CT scans [12]. The current NCCN guidelines for MM recommend that follow up imaging be used in cases of solitary plasmacytomas only if clarification is required for changes on the imaging studies noted at baseline or if there is suspicion of disease progression in the form of new onset bone pain or positive laboratory test results [12]. This strategy, while a prudent utilization of available resources, does not conform to the essence of recent trends in MM, where the goal has been to detect disease presence before it causes end organ damage [5]. In our patient, while the PET/CT scan was able to detect the EMPs on both occasions, the patient had no symptoms and was otherwise healthy at the time of the second occurrence.
PET/CT scans are currently considered a standard of care diagnostic modality for MM and plasmacytomas by most insurance carriers as well as the Centers for Medicare and Medicaid Services (CMS) in the United States (US) [5]. While the exact out-of-pocket cost for a PET/CT scan to a patient may vary significantly across the US, several databases quote a wide range of costs for the technique ($1,500-$13,000) based on the insurance coverage, geographic location, etc., [13]. Considering the reported utility of PET/CT scans for monitoring MM [14] and the lack of data for similar benefits for plasmacytomas, this is an area that requires standardized guidelines, especially with a cost-benefit view.
Conclusion
The present case highlights the importance of imaging by PET/CT scans for patients with solitary plasmacytomas, and more importantly, it reflects the utility that this imaging modality can have for patient follow up and surveillance. While a PET/CT scan is almost universally performed to confirm the diagnosis of a solitary plasmacytoma, standardized guidelines need to be developed to use this and possibly other technique for patient surveillance.
GI = gastrointestinal
[1]. Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R, Solitary plasmacytoma of bone and asymptomatic multiple myelomaBlood 2000 96(6):2037-44. [Google Scholar]
[2]. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS, Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004British Journal of Haematology 2009 144(1):86-94. [Google Scholar]
[3]. Finsinger P, Grammatico S, Chisini M, Piciocchi A, Foa R, Petrucci MT, Clinical features and prognostic factors in solitary plasmacytomaBritish Journal of Haematology 2016 172(4):554-60. [Google Scholar]
[4]. Frassica DA, Frassica FJ, Schray MF, Sim FH, Kyle RA, Solitary plasmacytoma of bone: Mayo Clinic experienceInternational Journal of Radiation Oncology, Biology, Physics 1989 16(1):43-48. [Google Scholar]
[5]. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, International Myeloma Working Group updated criteria for the diagnosis of multiple myelomaThe Lancet Oncology 2014 15(12):e538-48. [Google Scholar]
[6]. Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Improved survival in multiple myeloma and the impact of novel therapiesBlood 2008 111(5):2516-20. [Google Scholar]
[7]. Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytomaClinical Oncology 2004 16(6):405-13. [Google Scholar]
[8]. Dimopoulos MA, Hillengass J, Usmani S, Zamagni E, Lentzsch S, Davies FE, Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statementJournal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2015 33(6):657-64. [Google Scholar]
[9]. Bauerle T, Hillengass J, Fechtner K, Zechmann CM, Grenacher L, Moehler TM, Multiple myeloma and monoclonal gammopathy of undetermined significance: importance of whole-body versus spinal MR imagingRadiology 2009 252(2):477-85. [Google Scholar]
[10]. Lu YY, Chen JH, Lin WY, Liang JA, Wang HY, Tsai SC, FDG PET or PET/CT for detecting intramedullary and extramedullary lesions in multiple Myeloma: a systematic review and meta-analysisClinical Nuclear Medicine 2012 37(9):833-37. [Google Scholar]
[11]. Dammacco F, Rubini G, Ferrari C, Vacca A, Racanelli V, 18F-FDG PET/CT: a review of diagnostic and prognostic features in multiple myeloma and related disordersClinical and Experimental Medicine 2015 15(1):1-18. [Google Scholar]
[12]. NCCN clinical practice guidelines in oncology (Multiple Myeloma) [Internet]. National Comprehensive Cancer Network, Inc. 2016 [cited June 10, 2016]. Available from: https://http://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf [Google Scholar]
[13]. PET scan cost averages around the country [Internet]. New Choice Health, Inc. 2016 [cited June 10, 2016]. Available from: http://www.newchoicehealth.com/pet-scan-cost [Google Scholar]
[14]. Derlin T, Bannas P, Imaging of multiple myeloma: Current conceptsWorld Journal of Orthopedics 2014 5(3):272-82. [Google Scholar]