There are several methods to measure glycogen in animal tissues. The tissue is digested by hot alkaline [1,2], hot acid [3] or cold acid-grinding [4]. Then, glycogen is extracted from the (supernatant of) tissue homogenate with ethanol [5]. Glycogen is labile in hot acid and undergoes hydrolysis, so it cannot be extracted with ethanol in hot acid treatment [3]. Finally, glycogen is hydrolyzed to glucose and measured by using chemical or enzymatic methods [6,7].
The classical method was re-evaluated and optimized for assays of glycogen fractions [8]. In the classical homogenization method, the tissue is grounded by cold PCA. The extraction must be done at least twice to recover any ASG quantitatively [8–11]. The last pellet is digested with hot alkaline to release AIG. Total glycogen could be calculated by summing the values of ASG and AIG or measured directly by hot alkaline [1,2].
In homogenization-free method the tissue that is submerged in cold PCA is pressed by a plastic rod to the wall of the tube during 20 minutes [12,13]. The tissue suspension is then centrifuged, the supernatant is taken as ASG and the pellet is extracted by hot alkaline to remove AIG. We also introduced a new method in which total glycogen is extracted and divided directly to the fractions of ASG and AIG [14]. By using this procedure, glycogen fractions are measured in the same sample simultaneously. Recently, a new era of research has conducted to study the physiological role of glycogen fractions [15–18]. However, the results of the methods with and without of homogenization are completely different [11]. Therefore, it is essential to compare different methods for measuring glycogen fractions. The aim of the present study was to compare the results of different procedures to assay glycogen fractions. Accordingly, the fractions of glycogen were extracted and measured in rat liver at different physiological states by using three protocols: homogenization, homogenization-free and total-glycogen-fractionation.
Materials and Methods
The project was an animal experimental study that was performed on March of 2015 and continued for six months. Albino (Wistar) male rats weighing 200 gm-220 gm were housed in a room with 12 hour light/dark cycle under constant temperature (25°C) and humidity for 15 days. One group of five rats fed standard rodent laboratory food as controls, and another five rats were starved overnight (15 hour) as cases. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (GCULA) approved by National Research Council [8].
Liver Sampling
Diethyl ether was used to anaesthetize the rats, and then the liver was isolated from the animal and washed rapidly with ice cold isotonic saline three times. The lobs of the liver were incised into several parts on a filter paper and preserved between para-film plates at −70°C immediately [8].
Assay of Total Glycogen
A total of 50 mg of liver tissue was weighed and transferred to 200 μl 30% Potassium Hydroxide (KOH) and heated in boiling water bath for 10 minute with regular mixing. The sample was cooled and ethanol was added at a final concentration of 55%, the mixture was vortexed and centrifuged for 10 minute at 1700×g. The supernatant was decanted off and the pellet re-suspended in 2 ml of distilled water and 10 μl was analyzed for total glycogen in triplicate [8].
Classical Homogenization Method
The procedure was optimized previously [8], in brief 50-100 mg of liver tissue was weighed and transferred into a tube and ground 20 sec with 1 ml of ice cold 10% PCA using a tissue grinder (IKA works Ins., Wilmington, NC). To extract glycogen quantitatively from the tissue, the homogenizer probe was immersed, washed and homogenized with 1 ml fresh PCA for further five second and was added to the tissue homogenate. The ground sample was centrifuged for 10 minute at 280×g at 4°C. The supernatant containing ASG in suspension was decanted into another tube and the pellet was re-extracted for further one step with 1 ml fresh PCA to extract any ASG. To extract AIG, 200 μl of 30% KOH was added to the last pellet and heated in boiling water bath for 10 minute. After cooling, it was extracted with ethanol and centrifuged for 10 minute at 1700×g. The supernatant was decanted off and the pellet re-suspended in 1 ml of distilled water and 50 μl was analysed for AIG.
Total-Glycogen-Fractionation Method
According to the protocol of Rasoulis M et al., 30 μl of ice cold PCA (70%) was added to the cold suspension of total glycogen and mixed, the final pH was about three [14]. ASG was remained in the suspension while AIG was precipitated. The sample was centrifuged for five minute at 280×g. The supernatant that contains ASG in suspension was decanted into another tube. The pellet containing AIG was resolved in 2 ml of distilled water with help of 10 μl 30% of KOH, the final pH was about 9.5.
Homogenization-Free Method
The 50 or 100 mg tissue was weighed and transferred into a tube and was added 2 ml ice cold 10% PCA. The sample was pressed with a glass rod occasionally during 20 minute incubation in ice surrounding [12,18]. The ground sample was centrifuged for 10 minute at 280×g at 4°C. The supernatant containing ASG in suspension was decanted into another tube and the pellet was extracted by adding 200 μl of 30% KOH and heated in boiling water bath for 10 minute. After cooling, it was extracted with ethanol and centrifuged for 10 minute at 1700×g. The supernatant was decanted off and the pellet re-suspended in 1 ml of distilled water and 50 μl was analyzed for AIG.
Assay of Glycogen
The suspension of glycogen was mixed by vortex one second just before the sampling. Almost 10-50 μl of sample was used for the measurement of glycogen fractions by chemical method of phenol-sulfuric acid optimized previously [19,20].
Statistical Analysis
The results were presented as the means±SD of three inter-assays performed at least in five samples (Excel software: version 24.0. 2016). The significant differences between samples and corresponding control were checked by student’s t-test. All p-values are two-tailed and differences were considered significant if p-values were ≤0.05.
Results
Homogenization and total-glycogen-fractionation methods: To test and compare the results of different procedures, the fractions of glycogen were measured in liver by three methods in fed state and also following 15 hours starvation [Table/Fig-1a-c].
Liver glycogen fractions at fed and fast states. Two groups of five rats (n=5) were kept as fed and 15 hour fast and the liver was analyzed for glycogen fractions by using three procedures: (a) Classical homogenization; (b) total glycogen-fractionation; and (c) Homogenization–free method. All measurements were done in triplicate. *, ** and *** indicate significance at a confidence levels of p≤0.05, p≤0.01 and p≤0.001 respectively.
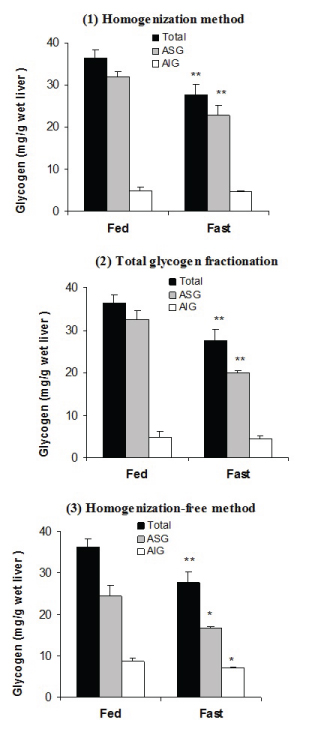
The data of homogenization method [Table/Fig-1a] shows that total glycogen was 36.4±1.9 (mg/g wet liver) in fed state and was decreased significantly to 27.7±2.5, p=0.01 following 15 hour starvation. ASG was the major portion of total glycogen in fed state (32.0±1.1) and was decreased to 22.7±2.5, p=0.01 during starvation. On the other hand, AIG was the minor portion of total glycogen (4.9±0.9) and did not change significantly after starvation (4.6±0.3, p=0.7). Similar results were obtained by using the ‘total glycogen fractionation’ procedure [Table/Fig-1b].
Homogenization-Free Method: The results of homogenization-free method are shown in [Table/Fig-1c]. The results of this procedure indicate that ASG and AIG fractions compromise about two-third and one-third of total glycogen respectively. Furthermore, the changes of the content of glycogen during starvation occurred in both ASG (24.4±2.6 vs. 16.7±0.4, p<0.05) and AIG fraction (8.7±0.8 vs. 7.1±0.3, p=0.05). The coefficient of variance (CV%) was less than 5% for all measurements (results not shown).
Discussion
In the current study, the fractions of glycogen were measured in rat liver at different physiological states by using three methods. The findings of ‘classical homogenization method’ indicated that ASG is the major portion of rat liver glycogen and is more metabolically active form [Table/Fig-1a]. The results were the same for ‘classical homogenization’ and ‘total-glycogen-fractionation methods’ as there were no any significant differences between their results [Table/Fig-1b]. The results obtained by using the ‘homogenization-free method’ were different as AIG had more contribution and also showed a significant change during starvation [Table/Fig-1c].
The ‘classical homogenization method’ was optimized in the previous study [8]. By using this method in the current study as well as in the previous report [8], total glycogen decreased during 15 hour fasting and the changes happened mainly in the soluble fraction of glycogen, while the insoluble fraction did not change significantly. This finding is clearly in accordance with the early studies that also used the same procedure [1–6], but is opposite to the results obtained by using recent homogenization- free protocols [12,13]. The ‘homogenization- free methods’ are encountered with three main problems; high relative error in weighting, incomplete homogenization and contamination of AIG with ASG. The high coefficient of variance CV% of the results obtained by this method is attributed to very small sample size 3-7 mg taken by biopsy. The current study did not encounter with this problem because using of 50 mg tissue sample. We showed in the previous study [8] that the yield of the recovery of ASG during successive extractions with cold PCA depends on the tissue concentration, and about 9% of ASG is remained in the second pellet at the ratio of 50 mg tissue per 2 ml PCA. In homogenization-free protocol, the extraction has been done only once by a glass rod followed by unnecessarily high speed centrifugation. Therefore, ASG is not extracted completely and some extracted ASG precipitates again during centrifugation causing a marked over-estimation of AIG. The increase in the time and extent of homogenization followed with ultrasonication also can not remove all glycogen on the first extraction [8]. Indeed, it does not difference to use homogenizer or pressing the tissue to the wall of the tube, in any way about 90% of ASG is removed during the first extraction. The second extraction is essential to extract ASG from the tissue quantitatively. As Barnes PD et al., mentioned [10,11], earlier studies that used a homogenization procedure have consistently reported more ASG than the recent studies without homogenization [1–7]. Therefore, in the homogenization-free protocols, some ASG contaminates AIG fraction and the changes that is seen in AIG is attributed to ASG fraction. In homogenization-free method, if the first pellet is re-extracted for ASG (by homogenization or pressing) with further 1 ml fresh cold-PCA and centrifuged with mild extent, the result of two methods will be identical (results not shown).
The values of ASG, AIG and total glycogen and the responses to starvation obtained by the protocol of Rasouli M et al., are the same as the classical method, but the procedure is more easy and precise [14]. The procedure avoided several extraction-centrifugation steps, hence, no any ASG is lost through successive extractions and less AIG is lost via autolysis. The time and extent of centrifugation has been chosen to be low in the fractionation step, so that no any ASG is co-precipitated with AIG [9]. In this method, the tissue is digested with hot alkaline to liberate total glycogen, then it is fractionated to ASG and AIG by adjusting the pH of the solution. Hot alkaline is used because any other procedure failed to extract total glycogen completely [20]. The criticism against this procedure is that glycogen may undergo hydrolysis during treatment with hot alkaline. But, the validity of the method is confirmed by two reasons. Firstly, the glycoside bonds (of glycogen) are not labile in hot alkaline [1,2,5]. Secondly, the results of this method are exactly the same as classical homogenization method.
Conclusion
The ASG fraction is the major portion of rat liver glycogen and is more metabolically active form. The results of ‘homogenization method’ are identical with ‘total glycogen fractionation’ procedure, but differ with the findings of ‘homogenization-free’ protocol. If the first pellet is re-extracted again with cold PCA for remaining ASG the results will be identical for the methods with and without homogenization.