Inferior Vena Cava Agenesis: A Rare Cause of Pelvic Congestion Syndrome
Satyendra Narayan Singh1, Trilok C Bhatt2
1 Surg Cdr and Associate Professor, Department of Radiology, Base Hospital, Delhi Cantt, New Delhi, India.
2 Surg Lt Cdr, Department of Radiology, INHS Kalyani, Visakhapatnam, Andhra Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Satyendra Narayan Singh, Surg Cdr and Associate Professor, Department of Radiology, Base Hospital, Delhi Cantt, New Delhi-110010, India.
E-mail: cdrsingh@gmail.com
Complete absence of Inferior Vena Cava (IVC) is a rare anomaly with a reported incidence of 0.0005% to 1%. This is often asymptomatic with incidental detection during cross-sectional imaging. It may also present with deep venous thrombosis, pulmonary thromboembolism or compressive symptoms in form of nerve root compression. Pelvic Congestion Syndrome (PCS) is an increasingly recognized entity with well laid out diagnostic criteria and evolving management protocols. Complete absence of IVC is a rare cause of pelvic congestion syndrome. We present a case of young female presenting with symptoms typical of pelvic venous congestion who was found to have complete absence of IVC as the underlying cause. She also had associated small left kidney with compensatory hypertrophy of the right kidney which is another rare association.
Atrophic left kidney, Subcardinal vein, Supracardinal vein
Case Report
A 28-year-old female presented with gradually worsening, non-cyclical pelvic pain and menorrhagia since last two years. No radiculopathy was present. The lady had three living children with the second pregnancy being twins delivered vaginally at full term. No history of any thrombolysis, intervention or surgery in the past. Clinical and gynaecological examinations were unremarkable. No lower limb, vulval or abdominal wall varicose veins were seen. An ultrasound of the abdomen and pelvis was done which showed non visualization of IVC replaced by multiple tortuous vascular channels. Small amount of free fluid was seen in the pouch of Douglas.
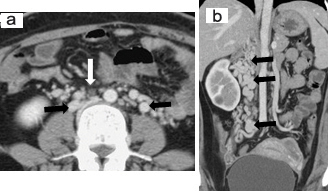
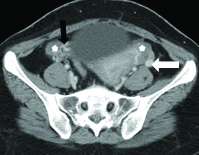
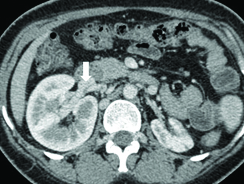
A Contrast Enhanced Computed Tomography (CECT) scan in venous phase of the chest, abdomen and pelvis was done. The scan revealed complete absence of the IVC as well as Common Iliac Veins (CIV) [Table/Fig-1]. The femoral veins were draining into external iliac veins which were in turn draining into multiple tortuous paravertebral venous channels. Both the ovaries were enlarged and showed multiple cysts within [Table/Fig-2]. The left gonadal vein was dilated measuring 11 mm in caliber. The right gonadal vein was replaced by multiple tortuous vascular channels. Tortuous vessels were also seen in the myometrium and in the pelvis [Table/Fig-2]. The IVC was also replaced by multiple tortuous vascular channels. Both renal veins were also draining into these vascular channels [Table/Fig-3]. These channels were communicating with paravertebral and ascending lumbar venous plexuses which were also dilated and tortuous [Table/Fig-4]. The venous drainage across diaphragm was via the azygous and hemiazygous veins both of which were prominent. The hepatic veins were seen to unite and drain directly into the right atrium [Table/Fig-5a]. The azygous vein was seen to drain into Superior Vena Cava (SVC) after arching over the right main bronchus [Table/Fig-5b]. The left kidney was small in size with hypertrophy of the right kidney. This was likely an incidental finding with no obvious intervention or surgery in the past.
a) Axial section at the level of infra renal aorta and coronal reconstruction (white arrow); b) Shows non visualization of IVC with multiple tortuous vascular channels seen in the para aortic region (Black arrows). Few of these channels show thrombus within.
Axial section at the level of uterus shows multiple tortuous vessels replacing the right ovarian vein (black arrow), the left ovarian vein is dilated (white arrow) and both ovaries are prominent and cystic (stars). Tortuous vascular channels are also seen around the uterus.
Axial section at the level of kidneys shows the right renal vein communicating with the tortuous vascular channels; the left renal vein was also seen to communicate with these channels (not shown). Also, seen is atrophic left kidney with enlarged right kidney. IVC is not seen in this images (left to right).
Axial section at the level of aortic bifurcation shows absent common iliac veins. Dilated tortuous veins are seen adjacent to right common iliac artery (white arrow).The external Iliac veins are absent bilaterally.

(a) Axial section at the level of superior margin of liver shows hepatic veins draining separately into the right atrium (black arrow). The prominent azygous vein can also be seen on the right side of aorta (white arrow). (b). Axial section at the level of aortic arch shows dilated azygous vein (white arrow) draining into the superior vena cava (star) Images (left to right).

Discussion
Chronic pelvic pain has been postulated to account for 10%-15% of gynaecological outpatient visits with majority of patients being in the age group of 20-45 years [1]. The association of chronic pelvic pain with pelvic varices was first documented in 1949 [2]. Various obstructing vascular anomalies have been documented to cause pelvic varices and include retro aortic left renal vein compressing the left ovarian vein leading to symptomatic pelvic varices, compression of the left ovarian vein and left renal vein by the superior mesenteric artery (Nutcracker phenomenon) and compression of the left common iliac vein by the right common iliac artery against the spine and pelvic brim (May- Thurner syndrome) [3]. The association between absence of IVC and pelvic varices causing PCS has been documented only in occasional case reports [4,5] primarily due to rarity of the condition.
Congenital anomalies of the IVC are increasingly being encountered since the advent of cross-sectional imaging. Complete absence of the IVC is a rare anomaly with an incidence of 0.0005% to 1% [6].
The IVC develops as a composite structure from a complex process of appearance and subsequent regression and fusion of three paired veins from the cardinal system (posterior cardinal, subcardinal and supracardinal veins in order of appearance) between sixth and eighth week of gestation [7].
Any variations in this basic plan lead to various observed anomalies of IVC. Explaining complete absence of IVC based on this model has been controversial as complete absence of IVC will require failure of development of all three cardinal venous systems. Perinatal IVC thrombosis has been suggested as a possible mechanism to explain [8].
PCS refers to chronic pelvic pain caused by congestion of the ovarian and pelvic veins due to valvular insufficiency and is more commonly seen in multiparous females [3,9]. Congenital anomalies of the venous system leading to obstruction of venous drainage are a well-recognized cause of this condition. Well laid out imaging criteria have been established for diagnosing this condition [3,10] which include: (a) Dilated ovarian veins measuring >4 mm in caliber; (b) Dilated tortuous arcuate veins in the myometrium communicating with bilateral pelvic varicose veins; (c) Slow blood flow (less than 3 cm/s), and reversed caudal or retrograde venous blood flow particularly in the left ovarian vein.
In our patient the left ovarian vein measured 11 mm in caliber. The right ovarian vein was replaced by multiple tortuous vascular channels and the myometrium also showed multiple serpiginous vascular structures within. An association of cystic ovaries has been seen in 50% of cases with PCS [11] possibly due to oestrogen overstimulation. In our patient also both ovaries were enlarged and showed multiple cysts within.
Absence of IVC leads to development of multiple collaterals predominantly via four large routes: the gonadal venous system, the paravertebral venous plexus, the haemorrhoidal plexus, and the superficial pathway through superficial abdominal veins. All these pathways ultimately drain into the SVC or the portal venous system (haemorrhoidal plexus).
The association of absence of IVC with renal anomalies is well documented and involves the right kidney in majority of cases. Renal agenesis, aplasia and hypoplasia have all been documented with the postulated mechanism being impaired venous drainage of right metanephros [9,12,13]. The involvement of left kidney is seen less frequently possibly due to alternate venous drainage of left metanephros via gonadal vein and lumbar perforators and has been documented in few case reports only [13,14]. The association between absence of IVC, renal anomalies and leg thrombosis has been termed Kidney and IVC abnormalities and Leg Thrombosis (KILT) syndrome [13]. Our patient had atrophic left kidney with compensatory hypertrophy of the right kidney; however, there was no thrombosis in the leg veins.
Most of patients with absence of IVC are asymptomatic and the anomaly is detected incidentally during imaging done for other purposes. The most common presentation is with lower limb Deep Venous Thrombosis (DVT). An association between IVC anomalies and increased risk of lower limb DVT has been postulated [15–17] and IVC anomalies should be actively looked for particularly in young patients with DVT. Other less frequently presentations include; sciatic neuropathy caused by dilated epidural veins compressing the lumbar nerve roots [18,19]; compressive symptoms in form of obstructive pyelonephritis [19], pelvic congestion syndrome in females and varicocele in males.
Cross-sectional imaging plays a vital role in diagnosis of absence of IVC and other IVC anomalies. CECT scan with image acquisition in venous phase as well as contrast enhanced MRI provide information about the exact type of anomaly as well as define the extent of venous thrombosis and its compressive effect. Catheter guided direct venography via femoral route is widely used diagnostic test; however it fails to define the thrombosed veins and the compressive effects.
The mainstay of treatment is conservative with anticoagulation to prevent venous thrombosis [20]. Surgical thrombectomy is indicated in cases with acute DVT [10,21]. Isolated case reports of endovascular reconstruction of the interrupted segment [22] or prosthetic graft placement for IVC reconstruction [23] exist without long term results.
Conclusion
Complete absence of IVC is a rare entity with varied presentation. Our patient presented with chronic pelvic pain and features of pelvic congestion syndrome. Cross-sectional imaging plays a vital role in diagnosis of absence of IVC and other associated developmental variations. CECT with imaging in venous phase and CEMRI provide anatomical variation in details. The mainstay of treatment is anticoagulation therapy. Surgical thrombectomy, endovascular reconstruction, prosthetic graft placement for IVC reconstruction are other modalities of treatment.
Informed consent: Informed consent was obtained from all individual participants included in the study.
[1]. Robinson JC, Chronic pelvic painCurr Opin Obstet Gynecol 1993 5:740-43. [Google Scholar]
[2]. Taylor HC, Vascular congestion and hyperemia: their effects on structure and function in the female reproductive systemAm J Obstet Gynecol 1949 57:637-53. [Google Scholar]
[3]. Ignacio EA, Ruchika D, Shawn S, Pelvic congestion syndrome: diagnosis and treatmentSemin Intervent Radiol 2008 25:361-68. [Google Scholar]
[4]. Nichols JL, Gonzalez SC, Bellino PJ, Bieber EJ, Venous thrombosis and congenital absence of IVC in a patient with menorrhagia and pelvic painJ Pediatr Adolesc Gynecol 2010 23(1):e17-21. [Google Scholar]
[5]. Wei Z, Wade R, Alan L, Li J, Successful surgical management of pelvic congestion and lower extremity swelling owing to absence of infrarenal inferior vena cavaVascular 2005 13(6):358-61. [Google Scholar]
[6]. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr, Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findingsRadio Graphics 2000 20:639-52. [Google Scholar]
[7]. Bass JE, Redwine MD, Kramer LA, Harris JH Jr, Absence of the infrarenal inferior vena cava with preservation of the suprarenal segment as revealed by CT and MR venographyAJR Am J Roentgenol 1999 172:1610-12. [Google Scholar]
[8]. Ramanathan T, Michael T, Hughes D, Richardson JA, Perinatal inferior vena cava thrombosis and absence of the infrarenal inferior vena cavaJournal of Vascular Surgery 2001 33:1097-99. [Google Scholar]
[9]. Gil RJ, Pérez AM, Arias JB, Pascual FB, Romero ES, Agenesis of the inferior vena cava associated with lower extremities and pelvic venous thrombosisJournal of Vascular Surgery 2006 44:1114-16. [Google Scholar]
[10]. Sagban TA, Grotemeyer D, Balzer KM, Tekath B, Pillny M, Grabitz K, Surgical treatment for agenesis of the vena cava: A single center experience in 15 casesEur J Vasc Endovasc Surg 2010 40:241-45. [Google Scholar]
[11]. Coakley FV, Varghese SL, Hricak H, CT and MRI of pelvic varices in womenJ Comput Assist Tomogr 1999 23:429-34. [Google Scholar]
[12]. Gayer G, Zissin R, Strauss S, Hertz M, IVC anomalies and right renal aplasia detected on CT: a possible link ?Abdominal Imaging 2003 28:395-99. [Google Scholar]
[13]. Veen J van, Hampton KK, Makris M, KILT syndrome?British Journal of Hematology 2002 118(4):1199-200. [Google Scholar]
[14]. Lawless RA, Dangleben DA, Caval agenesis with a hypoplastic left kidney in a patient with trauma on warfarin for deep vein thrombosisVascular and Endovascular Surgery 2012 46(1):75-76. [Google Scholar]
[15]. Ruggeri M, Tosetto A, Castaman G, Rodeghiero F, Congenital absence of the inferior vena cava: a rare risk factor for idiopathic deep-vein thrombosisLancet 2001 357:441 [Google Scholar]
[16]. Guanella R, Glauser F, Bounameaux H, Mazzolai L, Inferior vena cava agenesis: association with bilateral lower-limb deep vein thrombosis in young malesThromb Haemost 2009 102:795-98. [Google Scholar]
[17]. Obernosterer A, Aschauer M, Schnedl W, Lipp RW, Anomalies of the inferior vena cava in patients with iliac venous thrombosisAnn Intern Med 2002 136:37-41. [Google Scholar]
[18]. Kara M, Eken G, Zen GO, Kiraz S, Deep venous thrombosis and inferior vena cava agenesis causing double crush sciatic neuropathy in Behcet’s diseaseJoint Bone Spine 2008 75:734-36. [Google Scholar]
[19]. Xavier Y, Beatriz A, Elisabeth F, Miriam B, Matas M, Compressive symptoms due to thrombosed or hypertrophic collateral circulation in infrarenal Inferior Vena Cava AgenesisAnn Vasc Surg 2013 27(2):238.e9-238.e13. [Google Scholar]
[20]. Lambert M, Marboeuf P, Midulla M, Trillot N, Beregi JP, Mounier-Vehier C, Inferior vena cava agenesis and deep vein thrombosis: 10 patients and review of the literatureVasc Med 2010 15:451-59. [Google Scholar]
[21]. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis 9th edition. American college of chest physicians evidence-based clinical practice guidelinesChest 2012 Dec 142(6):1698-1704. [Google Scholar]
[22]. Thomas SD, Ofri A, Tang T, Englund R, Endovascular reconstruction of an interrupted inferior vena cavaInternational Journal of Surgery Case Reports 2014 5:59-62. [Google Scholar]
[23]. Dougherty MJ, Calligaro KD, DeLaurentis DA, Congenitally absent inferior vena cava presenting in adulthood with venous stasis and ulceration: A surgically treated caseJ Vasc Surg 1996 23:141-46. [Google Scholar]