Introduction
C1q play an important role in clearance of immune complexes and apoptotic cell debris. Impaired clearance leads to exposure of C1 native antigen and development of anti-C1q antibody formation. Anti-C1q antibody is well studied in Systemic Lupus Erythematosus (SLE). Significance of anti-C1q Ab in Indian SLE patients and their clinical manifestations is not clear.
Aim
The aim of this study was to investigate associations between anti-C1q antibody and clinical as well as serological markers of SLE.
Materials and Methods
Retrospective study of SLE patients fulfilling either American College of Rheumatology (ACR) 1990 or Systemic Lupus International Collaborating Clinics (SLICC) 2012 classification criteria were recruited from inpatients and outpatients services of the Clinical immunology and Rheumatology Department, Christian Medical College at Vellore, India between March 2013 and January 2015. Anti-C1q antibody was assayed by ELISA (Demeditec Diagnostics GmbH, Germany). Logistic regression analysis was performed to find the association of anti-C1q antibodies with serological and clinical parameters in SLE including Lupus Nephritis (LN).
Results
Sixty nine patients (54.76%) out of 126 SLE patients had LN. Anti-C1q levels were higher in patients with LN as compared to those without (p<0.05). Anti-C1q antibody was also significantly associated with positive C1q immunofluorescence staining in renal biopsy specimens (p<0.05). Overall, renal Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) {OR 1.35 (1.08-1.69)}, low C4 {OR 3.11 (1.04-9.26)} and mucocutaneous manifestation {OR 4.72 (1.38-16.05)} were independently associated with anti-C1q levels in serum.
Conclusion
Renal SLEDAI, low C4 and mucocutaneous manifestations were independently associated with raised anti C1q antibody in SLE patients.
Introduction
SLE is a chronic autoimmune disease characterised by multi organ manifestations. LN has been reported in less than 50% of SLE patients from Asia and this serious complication is associated with substantial morbidity and mortality [1,2].
The initial complement component C1q activates classical complement pathway and plays an important role in the clearance of immune complexes and apoptotic cell debris [1]. C1q specifically binds to early apoptotic cells and initiates complement activation in order to clear dying cells [2,3]. Impaired clearance of apoptotic cells leads to exposure of neo epitopes in collagen like region of C1 which forms the binding site for anti-C1q IgG antibody [2,4]. This binding results in augmentation of complement activation. Anti-C1q antibody is seen in hypocomplementemic urticarial vasculitis syndrome (100%), mixed connective tissue disorder (94%), Felty’s syndrome (76%), SLE (30-60%) and Rheumatoid vasculitis (32%) [5]. C1q deficiency-associated SLE/SLE-like disease is known to present commonly with discoid rash and oral ulcers, whereas arthritis is a less common feature in this subset [6].
Anti-C1q antibody is present in approximately one third of patients with SLE, especially in those with high disease activity and renal involvement [7]. Anti-C1q Ab can predict renal flare. Hence, anti-C1q Ab can be used as a biomarker for monitoring patients with LN [8–11]. There are also few reports showing no association between anti-C1q antibodies and LN [12,13]. Currently no clear explanations are known for these discrepant data on clinical associations of anti-C1q antibody. Genetic susceptibility and ethnicity can influence anti-C1q antibody [14,15]. Anti-C1q antibody is also more common in Asians as compared to Caucasians and African Americans. Levels of anti-C1q antibody is reported to be higher in younger SLE patients with age below 35 years [15]. Given the high incidence of LN and younger age of onset in Asian lupus patients, it is likely that our patients have high anti-C1q antibodies [16,17]. The aim of this study, therefore, was to find out any association between anti-C1q antibody and other laboratory markers as well as clinical features in our patients with SLE.
Materials and Methods
This retrospective study was carried out using laboratory and electronic records of our SLE patients attending outpatient and inpatient services of the Department of Clinical Immunology and Rheumatology between March 2013 and January 2015. Hospital data of patients fulfilling ACR 1990 or SLICC 2012 classification criteria for SLE who underwent anti-C1q antibody test during this period, were retrieved from laboratory register. Relevant clinical, laboratory and serological parameters corresponding to the time of anti-C1q assay were noted from hospital electronic medical record. Clinical parameters noted included presence of organ system involvement (e.g., arthritis, skin manifestations, serositis, and central nervous system involvement), thromboembolic events, major infections as well as demographic features like disease duration prior to presentation. Laboratory findings from hospital electronic medical records were also noted including ESR, haemoglobin, blood counts, complement C3 and C4, Urine Protein/Urine Creatinine ratio (UP/UC), presence of autoantibodies (like anti-dsDNA, anti nucleosome antibody and antiphospholipid antibodies) and biopsy results. Presence of lupus anticoagulant or anti cardiolipin antibody in our SLE patients was considered indicative of positive antiphospholipid antibody status. When other laboratory test results were not available at the precise date of anti-C1q antibody assays, test results within 15 days of the anti-C1q antibody measurement were accepted as concurrent test results [18]. Disease activity score was calculated by SLEDAI using the relevant data from the electronic record of the hospital at the time of anti-C1q assay. Accordingly, disease activity in all SLE patients was classified as mild, moderate, or severe, based on their SLEDAI scores (mild <8, moderate 8-18, and severe >18). Renal SLEDAI score was calculated to assess kidney disease activity as described earlier [19].
Blood samples for anti-C1q antibody assay were collected from patients during their hospital visits irrespective of disease duration or dose of immunosuppressants. Anti-C1q Antibody was assayed by commercially available ELISA kit (Demeditec Diagnostics GmbH, Germany). Results were expressed as unit/ml (U/ml) and serum level more than or equal to 10 U/ml (cut off value), was considered positive, as recommended by the manufacturer.
This study was approved by the Institutional Review Board (ethics and research committee) of Christian Medical College, Vellore, Tamil Nadu, India.
Statistical Analysis
Continuous data are presented as mean (SD) or median (range) and categorical data are presented as frequency. Mann-Whitney test was performed to find out any significant difference of anti-C1q Ab titer between: a) Patients with and without LN; and b) between patients with active LN and Inactive LN. To identify any association of clinical and laboratory variables with anti-C1q antibody, univariate logistic regression analysis was done. Organ involvement, major infection and thromboembolic events were categorised as present or absent at the time of anti-C1q antibody assay. Laboratory variables included C3 and C4 levels, ESR and urine protein/urine creatinine ratio, and these were categorised as low or normal whereas anti- dsDNA antibody, anti nucleosome antibody and anti phospholipid antibodies were categorised as positive or negative. The relevant variables with p<0.05 by univariate logistic regression analysis were subjected to multivariable logistic regression analyses. A two tailed p-value of <0.05 was considered as significant. The statistical analysis of data was done using STATA 13.1 (StataCorp LP, Texas, USA) statistical package.
Results
Baseline characteristics of 126 patients included in the study are shown [Table/Fig-1]. LN was present in 54.76% of the patients. Anti-C1q antibody was present in 42.85% of the patients. Anti-C1q levels were significantly higher in patients with LN, as compared to lupus patients without LN (p<0.05) [Table/Fig-2a]. Similarly, anti-C1q levels were significantly higher in patients with active LN, as compared to inactive LN (p<0.05) [Table/Fig-2b].
Patients characteristics.
| Parameters | Total number of patients (n=126) |
|---|
| Age in years - Median(range) | 28 (13-62) |
| Male: Female (%) | 11 (8.73%):115 (91.26%) |
| Lupus nephritis | 69 (54.76%) |
| Serositis | 7 (5.6%) |
| Arthritis | 46 (36.5%) |
| Mucocutaneous manifestation | 51 (40.47%) |
| Neuropsychiatric manifestation | 17 (13.5%) |
| Major Infection | 5 (3.9%) |
| Thromboembolic events | 6 (4.7%) |
| Median disease duration at presentation in months (range) | 6 (1-96) |
| Median serum creatinine | 0.72 (0.1-2.21) |
| Median 24 hour urinary protein (mg) | 283 (99.5-949.5) |
| Nephrotic range proteinuria (n) | 6/126 (4.76%) |
| Impaired renal functions (n) | 4/126 (3.17%) |
| Systemic arterial hypertension (n) | 5/126 (3.96%) |
| Median SLEDAI (range) | 6 (0-29) |
| Mean Renal SLEDAI (range) | 1.7 (0-16) |
| Median C1q Ab titers | 6 (0-114.9) |
| Presence of autoantibodiesAnti-C1q antibodyAnti nucleosome antibodyanti-dsDNA antibodyanti phospholipid antibodies | n/N* (%)54/126 (42.85%)46/91 (50.54%)84/125 (67.2%)30/106 (28.3%) |
*n represents the number of patients in whom the test results were available.
Scatter plot show anti-C1q Ab levels; (a). Between patients with LN (n-69) and without LN (n-57); (b). Between patients with active LN (n-32) and Inactive LN (n-37)
*Mann-Whitney U test was used.
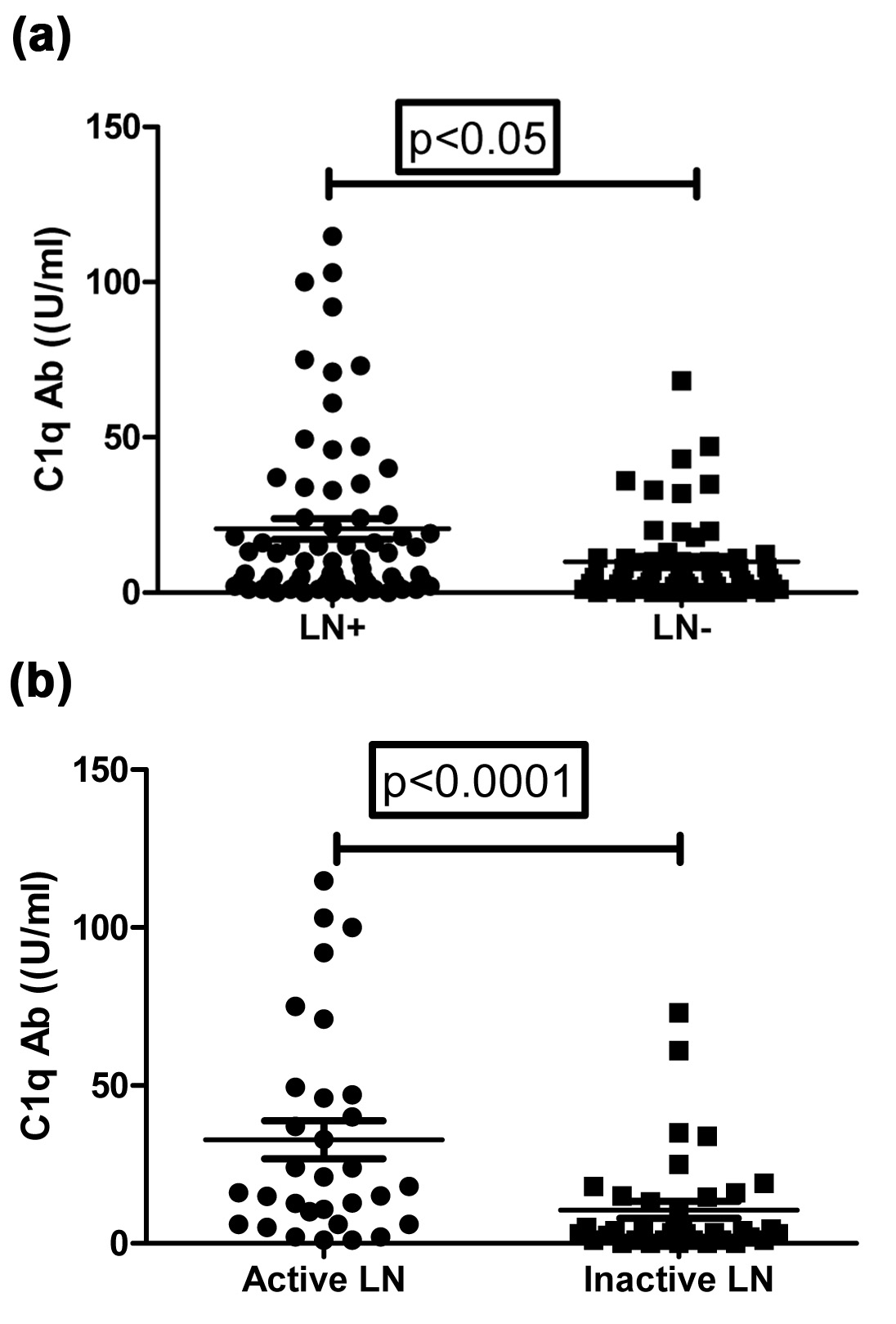
Association of Anti-C1q Antibody in all SLE Patients
Results of univariate analysis are shown [Table/Fig-3]. Variables found to be significant in univariate analysis were subjected to multivariate logistic regression with the exception of anti nucleosome antibodies and SLEDAI [Table/Fig-3]. Anti nucleosome antibody tests were available only for 91 patients, hence it was excluded from multivariable analysis. SLEDAI was also excluded from multivariate analysis, as it encompasses some of the other clinical and lab variables used in analysis. In multivariate analysis, low C4 (OR 3.11), mucocutaneous features (OR 4.72) and higher renal SLEDAI (OR 1.35) were found to be significantly associated with positive anti-C1q antibody. On removing renal SLEDAI from analysis, UP/UC {OR 1.77(0.63-4.95), (p<0.05)} also attained significance.
Associations of anti- C1q antibodies with clinical manifestations and laboratory parameters in SLE patients including lupus nephritis (univariate analysis and multivariate analysis).
| Parameters | Univariate logistic regression analysis | Multivariate logisticregression analysis |
|---|
| Odds ratio(95% CI) | p-value | Odds ratio(95% CI) | p-value |
|---|
| Anti nucleosome Abs | 6.57 (2.63-16.43) | 0.001* | ND | -ND |
| Anti-dsDNA | 4.1 (1.74-9.64) | 0.01* | 1.34 (0.44-3.82) | 0.629 |
| Antiphospholipid Abs | 0.74 (0.31-1.74) | 0.493 | ND | ND |
| Low C3 | 4.5 (2.09-9.68) | 0.001* | 1.37 (0.44-4.28) | 0.581 |
| Low C4 | 4.95 (2.3-10.67) | 0.001* | 3.11 (1.04-9.26) | 0.05* |
| ESR | 1.69 (0.71-3.99) | 0.230 | ND | ND |
| Haemoglobin | 0.91 (0.75-1.1) | 0.343 | ND | ND |
| Leucopenia | 0.966 (0.48-1.94) | 0.923 | ND | ND |
| UP/UC | 4.43 (2-9.77) | 0.001* | 1.77 (0.63-4.95) | 0.276 |
| Serositis | 2.12 (0.56-7.93) | 0.262 | ND | ND |
| Arthritis | 1.51(0.69-3.3) | 0.292 | ND | ND |
| Mucocutaneous | 2.65 (1.12-6.31) | 0.05* | 4.72 (1.38-16.05) | 0.05* |
| SLEDAI-Moderate | 6.33 (2.28-17.3) | 0.001* | ND | ND |
| SLEDAI-Severe | 17.4 (5.34-56.7) | 0.001* | ND | ND |
| Renal SLEDAI | 1.57 (1.29-1.92) | 0.001* | 1.35 (1.08-1.69) | 0.01* |
*ND- Not Done.
Association of Anti-C1q Antibody in Lupus Nephritis
We also did a separate univariate analysis with laboratory markers and clinical features in LN patients (n-69) [Table/Fig-4]. Anti-nucleosome antibody, anti-dsDNA antibody, low C3 and low C4, high UP/UC and renal SLEDAI were found to be significantly associated with anti-C1q antibody. No significant association was found when these parameters underwent multivariate logistic regression.
Associations of anti- C1q antibodies with clinical manifestations and laboratory parameters in SLE patients with LN as revealed by univariate logistic regression.
| Parameters | Odds ratio (95% CI) | p-value |
|---|
| Anti nucleosome Abs | 10.68 (2.88-39.62) | 0.001* |
| Anti-dsDNA | 6.25 (1.79-21.77) | 0.005* |
| Antiphospholipid Abs | 0.73 (0.21-2.5) | 0.627 |
| C3 | 8.17 (2.63-25.32) | 0.001* |
| C4 | 5.01 (1.79-14.05) | 0.005* |
| ESR | 3.72 (1.34-10.24) | 0.068 |
| Haemoglobin | 1.06 (0.82-1.39) | 0.621 |
| Leucopenia | 0.66 (0.25-1.72) | 0.398 |
| UP/UC | 3.71 (1.34-10.24) | 0.05* |
| Serositis | 1.38 (0.28-6.68) | 0.691 |
| Arthritis | 2.91 (0.79-6.07) | 0.131 |
| Mucocutaneous | 1.35 (0.45-4) | 0.583 |
| Renal SLEDAI | 1.52 (1.2-1.92) | 0.001* |
*Significant at 5% level of significance.
Discussion
Anti-C1q antibody is associated with SLE, in particular with LN. The result of present study on associations of anti-C1q antibody with LN in Asian Indian population is consistent with previous studies reported in western as well as Asian population. Multivariate analysis didn’t reveal any significant association of anti-C1q antibody in LN. In the whole SLE cohort, however, low C4, mucocutaneous and renal SLEDAI were independently associated with elevated serum anti-C1q antibody levels.
The frequency of anti-C1q antibodies in all SLE patients and LN subgroup were 42.3% and 50.7%, respectively in the present study as compared to 58.3% and 60%, respectively in a study from Western India [20]. The difference in frequencies could be accounted by larger sample size in our study. The prevalence of anti-C1q in another study consisting of multiethnic SLE patients was 28%, which is lower than the present study. In our study, anti-C1q antibody levels were significantly higher in patients with LN as compared to lupus patients without LN (p<0.05), which has not been reported previously [20–22]. Association of anti-C1q Ab with anti nucleosome Ab, anti-dsDNA Ab and urine protein/creatinine ratio in LN patients was not established in our multivariate analysis, possibly due to relatively lesser number of subjects with LN. Our finding of a pronounced association of anti-C1q antibody with anti nucleosome antibody and also a fairly strong association with anti dsDNA antibody may be explained by plausible biological basis. Nucleosome enhances binding of C1q in glomerular endothelial cells undergoing apoptosis and this interaction leads to potential binding of anti-C1q autoantibodies [23]. Anti-C1q antibody is considered to be predictor of active LN and in our study too, we found higher levels in active disease, as compared to inactive LN, similar to observation by others [24,25]. However, some reports also suggest that SLE patients negative for anti-C1q antibody are unlikely to get LN [10,26].
Association of renal SLEDAI with anti-C1q antibody observed in our study confirms utility of this antibody as a biomarker for renal involvement in SLE [15]. In the multivariate analysis, once renal SLEDAI was eliminated, UP/UC ratio {OR 1.77(0.63-4.95)} was also a significant association of anti-C1q antibody. This may imply that UP/UC levels are an important contributing factor towards renal SLEDAI in lupus nephritis patients with high levels of anti-C1q antibodies. This is again consistent with previous findings showing association of anti-C1q antibody with proteinuria and renal disease activity [8,27]. Further, combination of anti-C1q antibody and anti- dsDNA antibody predicts poorer renal outcome indicating active renal disease [28]. Results from this study also may suggest that anti-C1q antibody in combination with anti nucleosome antibody can be a better reflector of active LN.
In our SLE cohort, anti-C1q antibody is also highly associated with mucocutaneous involvement {OR 4.72 (1.38-16.05)}. Data on association of cutaneous features with anti-C1q antibody is scarce in SLE literature [29]. Bălănescu E et al., reported that SLE patients had increased titers of anti-C1q in serum [30]. Anti-C1q antibodies decrease complement proteins in circulation including C1q [31]. Congenital deficiency of C1q have been observed in early onset photosensitive SLE [32]. Although, low C1q in adult SLE patients is an acquired phenomenon caused by anti-C1q antibodies, could this have produced cutaneous manifestations as in patients with congenital C1q deficiency?
Nevertheless, association of early onset SLE with deficiency of C1q is well known [33]. In our study also, anti-C1q antibody levels were different between age groups with a cut off of 35 years of age; although this did not reach statistical significance, the trend was visible (p=0.055). Orbai AM et al., found that anti-C1q antibody level was higher in SLE patients with age below this age cut off [15]. These data support the notion that anti-C1q antibodies were more common in younger onset SLE patients.
Limitation
Limitation of this study includes retrospective design and smaller sample size.
Conclusion
In conclusion, low complement protein C4, mucocutaneous features and higher renal SLEDAI were independently associated with high anti-C1q antibody titre. Anti-C1q antibody, therefore, may act as a surrogate marker of renal and cutaneous involvement in SLE.
*n represents the number of patients in whom the test results were available.
[1]. Walport MJ, ComplementN Engl J Med 2001 344:1058-66. [Google Scholar]
[2]. Bigler C, Schaller M, Perahud I, Osthoff M, Trendelenburg M, Autoantibodies against complement C1q specifically target C1q bound on early apoptotic cellsJ Immunol 2009 183:3512-21. [Google Scholar]
[3]. Mevorach D, Clearance of dying cells and systemic lupus erythematosus: The role of C1q and the complement systemApoptosis Int J Program Cell Death 2010 15:1114-23. [Google Scholar]
[4]. Schaller M, Bigler C, Danner D, Ditzel HJ, Trendelenburg M, Autoantibodies against C1q in systemic lupus erythematosus are antigen-drivenJ Immunol Baltim Md 2009 183:8225-31. [Google Scholar]
[5]. Potlukova E, Kralikova P, Complement component C1q and anti-C1q antibodies in theory and in clinical practiceScand J Immunol 2008 67:423-30. [Google Scholar]
[6]. Stegert M, Bock M, Trendelenburg M, Clinical presentation of human C1q deficiency: How much of a lupus?Mol Immunol 2015 67(1):3-11. [Google Scholar]
[7]. Horak P, Skacelova M, Zadrazil J, Smrzova A, KrejcÍ K, Ciferska H, Complement system in SLE as a target for antibodiesCurr Rheumatol Rev 2013 9:34-44. [Google Scholar]
[8]. Moroni G, Quaglini S, Radice A, Trezzi B, Raffiotta F, Messa P, The value of a panel of autoantibodies for predicting the activity of lupus nephritis at time of renal biopsyJ Immunol Res 2015 2015:106904 [Google Scholar]
[9]. Abdel Kader MSEM, Abd Elaziz MM, Ahmed DH, Role of serum anti-C1q antibodies as a biomarker for nephritis activity in pediatric and adolescent Egyptian female patients with SLEExpert Opin Med Diagn 2012 6:489-98. [Google Scholar]
[10]. Mahler M, van Schaarenburg RA, Trouw LA, Anti-C1q autoantibodies, novel tests, and clinical consequencesFront Immunol 2013 4:117 [Google Scholar]
[11]. Edelbauer M, Kshirsagar S, Riedl M, Haffner D, Billing H, Tönshoff B, Markers of childhood lupus nephritis indicating disease activityPediatr Nephrol Berl Ger 2011 26:401-10. [Google Scholar]
[12]. Moura CG, Lima I, Barbosa L, Athanazio D, Reis E, Reis M, Anti-C1q antibodies: Association with nephritis and disease activity in systemic lupus erythematosusJ Clin Lab Anal 2009 23:19-23. [Google Scholar]
[13]. Trad B, Ben Hassine H, Khalifa M, Idriss N, Slama F, Bahri F, Anti-C1q antibodies and systemic lupus erythematosus in the Tunisian populationPathol Biol (Paris) 2013 61:113-16. [Google Scholar]
[14]. Pradhan V, Patwardhan M, Nadkarni A, Ghosh K, Fc y RIIA Genotypes and its association with anti-C1q autoantibodies in lupus nephritis (LN) patients from Western IndiaAutoimmune Dis 2010 2010:470695 [Google Scholar]
[15]. Orbai A-M, Truedsson L, Sturfelt G, Nived O, Fang H, Alarcón GS, Anti-C1q antibodies in systemic lupus erythematosusLupus 2015 24:42-49. [Google Scholar]
[16]. Samanta A, Feehally J, Roy S, Nichol FE, Sheldon PJ, Walls J, High prevalence of systemic disease and mortality in Asian subjects with systemic lupus erythematosusAnn Rheum Dis 1991 50:490-92. [Google Scholar]
[17]. Jakes RW, Bae S-C, Louthrenoo W, Mok CC, Navarra SV, Kwon N, Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: Prevalence, incidence, clinical features, and mortalityArthritis Care Res 2012 64:159-68. [Google Scholar]
[18]. Bock M, Heijnen I, Trendelenburg M, Anti-C1q antibodies as a follow-up marker in SLE patientsPLoS ONE 2015 10:e0123572 [Google Scholar]
[19]. Xuejing Z, Jiazhen T, Jun L, Xiangqing X, Shuguang Y, Fuyou L, Urinary TWEAK level as a marker of lupus nephritis activity in 46 casesBioMed Res Int 2012 2012:e359647 [Google Scholar]
[20]. Pradhan V, Rajadhyaksha A, Mahant G, Surve P, Patwardhan M, Dighe S, Anti-C1q antibodies and their association with complement components in Indian systemic lupus erythematosus patientsIndian J Nephrol 2012 22:353-57. [Google Scholar]
[21]. Zhang C-Q, Ren L, Gao F, Mu FY, You YQ, Liu YH, Anti-C1q antibodies are associated with systemic lupus erythematosus disease activity and lupus nephritis in northeast of ChinaClin Rheumatol 2011 30:967-73. [Google Scholar]
[22]. Oelzner P, Deliyska B, Fünfstück R, Hein G, Herrmann D, Stein G, Anti-C1q antibodies and antiendothelial cell antibodies in systemic lupus erythematosus -relationship with disease activity and renal involvementClin Rheumatol 2003 22:271-78. [Google Scholar]
[23]. O’Flynn J, Flierman R, van der Pol P, Rops A, Satchell SC, Mathieson PW, Nucleosomes and C1q bound to glomerular endothelial cells serve as targets for autoantibodies and determine complement activationMol Immunol 2011 49:75-83. [Google Scholar]
[24]. Marto N, Bertolaccini ML, Calabuig E, Hughes GR, Khamashta MA, Anti-C1q antibodies in nephritis: Correlation between titres and renal disease activity and positive predictive value in systemic lupus erythematosusAnn Rheum Dis 2005 64:444-48. [Google Scholar]
[25]. Chen Z, Wang G-S, Wang G-H, Li X-P, Anti-C1q antibody is a valuable biological marker for prediction of renal pathological characteristics in lupus nephritisClin Rheumatol 2012 31:1323-29. [Google Scholar]
[26]. Pickering MC, Botto M, Are anti-C1q antibodies different from other SLE autoantibodies?Nat Rev Rheumatol 2010 6:490-93. [Google Scholar]
[27]. Akhter E, Burlingame RW, Seaman AL, Magder L, Petri M, Anti-C1q antibodies have higher correlation with flares of lupus nephritis than other serum markersLupus 2011 20:1267-74. [Google Scholar]
[28]. Yang X, Tan Y, Yu F, Zhao MH, Combination of anti-C1q and anti-dsDNA antibodies is associated with higher renal disease activity and predicts renal prognosis of patients with lupus nephritisNephrol Dial Transplant 2012 27:3552-59. [Google Scholar]
[29]. Hegazy A, Barakat AF, El Gayyar MA, Arafa LF, Prevalence and clinical significance of anti-C1q antibodies in cutaneous and systemic lupus erythematosusEgypt J Med Hum Genet 2012 13:167-71. [Google Scholar]
[30]. Bălănescu E, Tănăsescu C, Bălănescu P, Olteanu R, Badea C, Petruţescu B, nti C1q antibodies in cutaneous lupus erythematosusRomanian J Intern Med Rev Roum Médecine Interne 2010 48:159-63. [Google Scholar]
[31]. Tan Y, Song D, Wu L, Yu F, Zhao M-H, Serum levels and renal deposition of C1q complement component and its antibodies reflect disease activity of lupus nephritisBMC Nephrol 2013 14:63 [Google Scholar]
[32]. Sontheimer RD, Racila E, Racila DM, C1q: Its functions within the innate and adaptive immune responses and its role in lupus autoimmunityJ Invest Dermatol 2005 125:14-23. [Google Scholar]
[33]. Al-Mayouf SM, Abanomi H, Eldali A, Impact of C1q deficiency on the severity and outcome of childhood systemic lupus erythematosusInt J Rheum Dis 2011 14:81-85. [Google Scholar]