TB is a major cause of mortality in developing countries accounting for over 1.5 million deaths per year. This disease is mainly caused by M.tuberculosis, an acid fast bacillus which spreads through the air [1]. WHO reported that TB killed 1.5 million people (1.1 million HIV-negative and 0.4 million HIV-positive). The toll comprised 8,90,000 men, 4,80,000 women and 1,40,000 children [2]. Indonesia is the number two among high burden countries, with estimate that there are about 1 million new TB cases per year, twice the previously estimated. Children under 15 years of age comprise 40%-50% of overall population, TB in children is of an important determinant in national TB program in developing countries [2].
Pulmonary TB is the most common type of TB in children. Pulmonary TB diagnosis is commonly based on clinical symptoms, chest X-ray, microbiology examination using sputum microscopy and culture. This is difficult in children because clinical presentation is not specific and chest X-ray interpretation has low accuracy and high inter observer variation [3]. Microbiology examination for M. tuberculosis is the gold standard for TB diagnosis, but this is not easy to perform in children due to difficulty in obtaining sputum sample and paucibacilary nature of the disease. Therefore, diagnosis is often based on clinical presentation of TB, history of contact with active adult patient, TST and response to anti TB medication. In Indonesia, a scoring system is developed for pulmonary TB diagnosis in children despite some limitation concerning its sensitivity and specificity [2–5].
WHO in 2011 banned the use of available and commercialized serologic tests for TB diagnosis due to inconsistence and imprecise accuracy and specifity of those tests. The policy encourages further studies to develop new serologic test for children with TB based on antigen biomarker [6].
TB antigen detection using ELISA or ICT method is one of the serologic techniques. M.tuberculosis antigen detection has an advantage reflecting the presence of mycobacterial material. Compared to sputum sample, detection of the antigen in the urine is more beneficial because urine sampling is easier and non-invasive [7].
Previous study show that LAM in urine and pulmonary specimen showed a sensitivity of 93% and a specificity of 95% [9]. Another study demonstrated the decline of urinary LAM concentration after two months treatment of anti TB in TB patients [8].
Urinary LAM detection could be promising alternative approach for rapid diagnosis of active TB. Urinary LAM detection performance is best in patient with disseminated TB and immunocompromised such as HIV patient [8,9].
The present study aimed to determine the diagnostic value of urinary LAM antigen in diagnosis of childhood TB.
Materials and Methods
Study Design and Patient Population
An observational, cross-sectional study was conducted between June, 2013 and June. 2014. Study population were paediatric patients aged 0-14 years at the Child Health Department of Saiful Anwar Hospital in Malang, East, Java, Indonesia that clinically suspected for pulmonary TB. There were 97 suspected patients between these period. Approval for study initiation was obtained from Faculty of Medicine Universitas Brawijaya/Saiful Anwar Hospital Ethical Commitee. Informed consent was obtained from either parents or guardian accompanying the patients.
Data Extraction
A total of 97 children aged 0-14 years were suffering from cough more than two weeks, fever without clear aetiology, loss of body weight or poor weight gain, fatigue, malaise, and have history of contact with positive sputum smear adult TB patient were enrolled to the study. Children with symptoms and signs of extra pulmonary TB, such as chronic cervical, submandibular and supraclavicular lymph node enlargement, spine angulation, joint swelling were also included. Subjects previously diagnosed to have pulmonary or extra pulmonary TB, had already received anti-TB medications for more than two weeks were excluded. Subjects were also excluded if leukocyturia, haematuria, and moderate to severe proteinuria reflecting renal abnormalities were found in urinalysis. [Table/Fig-1] depicts the flow chart of the study.
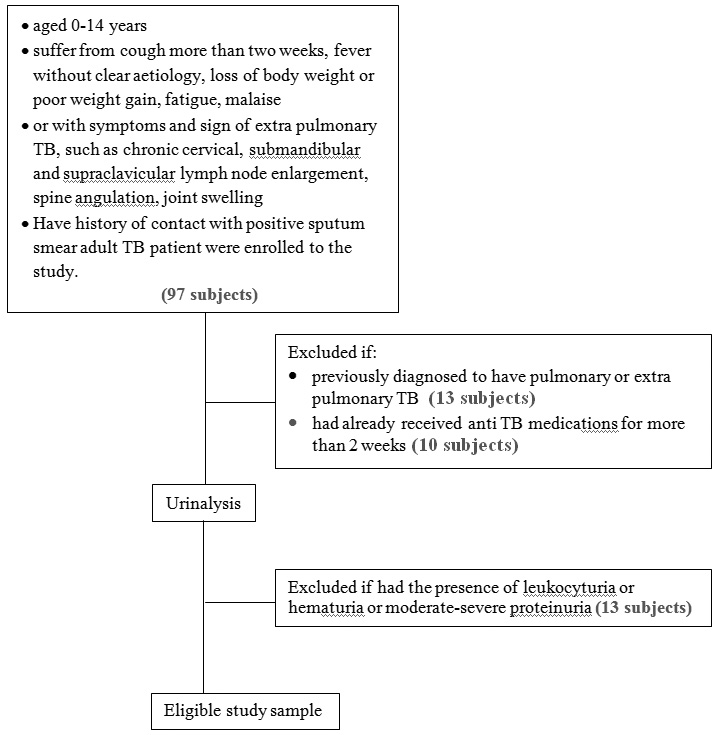
A thorough personal, family and medication history was taken to know if there was any household adult contact with active TB patient and any previous history of anti TB treatment. Physical examination was performed systematically including evaluation of nutritional status using WHO child chart standard. Subjects were categorized as malnourished if the body weight plotted under 2 z score for appropriate age [10].
Chest X-ray was interpreted by senior paediatric pulmonologist (MSC). Interpretation would be suggestive of TB or not. However, the result of chest X-ray would be considered in relation to overall clinical presentation.
TST were performed in all subjects using 2 TU PPD RT-23. The test considered positive if diameter of induration was greater than 5 mm in HIV infected subject and greater than 10 mm in HIV uninfected [11].
Urine sample (1-3 ml) was taken at first presentation. Routine urinalysis and LAM level measurement using ELISA method (Bioassay Technology Laboratory) [12] was performed. All subject had sputum examination for M.tuberculosis staining (Ziehl-Neelsen) and culture (Lowenstein Jensen). Sputum samples were obtained by gastric lavage or sputum induction. Histology examination were performed accordingly in 15 patients suspected for extrapulmonary TB.
Subjects were diagnosed pulmonary TB and considered microbiologically confirmed TB when at least one positive result of sputum staining or positive M.tuberculosis culture. When microbiology examination showed negative results, subjects were considered unconfirmed TB cases if they met at least two of the following symptoms and signs suggestive of pulmonary TB; chest radiograph consistent with TB; close TB exposure or positive TST; and noted positive clinical response after two months course of anti TB treatment.
HIV screening were offered to subjects diagnosed with both clinically or microbiologically confirmed TB. HIV infected patients suspected of having pulmonary TB had the same protocol as other subjects.
Statistical Analysis
Categorical variables were reported as frequencies and proportions, while quantitative variable (urine LAM antigen levels) were presented as mean±SD. Differences across categorical variables among the non-TB and TB groups was assessed using Pearson Chi-square test or Fischer-Exact test as appropriate, while differences in the mean urine LAM antigen level across the two groups were evaluated using an independent samples t-test.
We plotted various intersection points for sensitivity and specificity values to determine the cut off values. Positive Predictive Value (PPV), Negative Predictive Value (NPV), Positive Likelihood Ratio (PLR), and Negative Likelihood Ratio (NLR) were calculated from 2 x 2 table analysis.
All statistical analysis were performed using software SPSS 21.0. All tests were 2-tailed with a type I error set at 5%.
Results
Fourty nine (80.3%) of 61 subjects suspected of either pulmonary and extra pulmonary TB were eventually diagnosed with TB, in which 34 (69.8%) were pulmonary TB. Of those diagnosed with TB, 21 (42.9%) were microbiologically confirmed cases either by sputum microscopy (34.7%) or culture (8.2%), whereas, 28 subjects were unconfirmed cases (57.1%). Fifteen subjects were diagnosed with extra pulmonary TB which consist of TB meningitis (10%), TB lymphadenitis (6%), TB spondylitis and TB peritonitis (4% each), and TB pericarditis, urogenital, and coxitis (2% each). The characteristics of subjects were shown on [Table/Fig-2].
Characteristics of subjects.
| Characteristics | Non-TB (n = 12) | TB (n = 49) |
|---|
| Age (year) | 3.3±2.27 | 6.8±4.37 |
| 0-1 year | 1 (8.3) | 10 (20.4) |
| >1 – 5 year | 6 (50) | 9 (18.4) |
| >5 – 10 year | 5 (41.6) | 14 (28.6) |
| >10 year | 0 | 16 (32.7) |
| Male sex | 8 (66.7) | 28 (57.1) |
| HIV infection* | - | 3 (1.47) |
| Positive TST result | 0 | 24 (49) |
| Urinary LAM (mg/l) | 0.46±0.30 | 1.80±1.02 |
| Positive microbiology test | 0 | 21 (42.9) |
| Nutritional status | | |
| Normal | 9 (75) | 22 (44.9) |
| Malnourished | 3 (25) | 27 (55.1) |
Data was presented in N(%), and mean±SD *HIV examination was not performed in all subjects, it was done based on parental history with HIV
In [Table/Fig-2], urinary LAM level was higher in subjects with TB (1.80+1.02) mg/l compared to non-TB group (0.46+0.3) mg/l and statistically significant using independent t-test (p<0.001). Using SPSS 21.0 software, cut off value of urinary LAM level was 0.98 mg/l when compared to diagnosis of TB (including clinically and microbiology confirmed cases). When positivity of microbiology examination used as standard reference, cut off value of urinary LAM level was 1.69 mg/l.
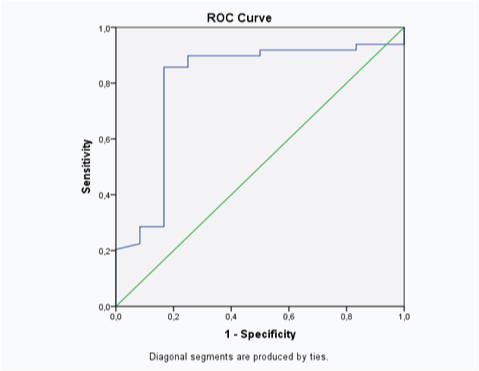
[Table/Fig-3] showed association of urinary LAM level and diagnosis of TB based on either clinical or microbiological confirmation. Based on this 2 x 2 table, the sensitivity of the test was 85.71%, specificity 83.3%, positive predictive value 55.45%, negative predictive value 58.22%, positive likelihood ratio 5.14, negative likelihood ratio 0.17 [Table/Fig-4].
A 2 X 2 table of urinary LAM level (cut off point 0.98 mg/dl) and diagnosis of TB.
| Urinary LAM | Diagnosis | Total |
|---|
| TB | Non TB |
|---|
| ≥ 0.98 mg/L | 42 | 2 | 44 |
| < 0.98 mg/L | 7 | 10 | 17 |
| Total | 49 | 12 | 61 |
ROC curve showed AUC of this test was 0.8 (95%CI 0.64 – 0.96).
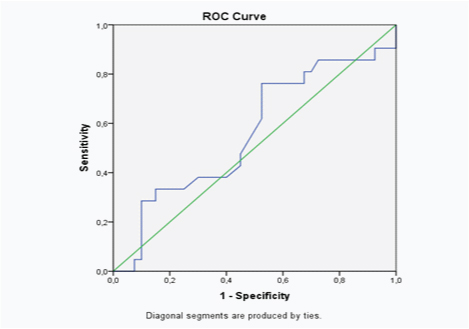
[Table/Fig-5] showed association of urinary LAM level and positivity of microbiology examination. Based on this 2 x 2 table, the sensitivity of the test was 33%, specificity 60%, positive predictive value 30.43%, negative predictive value 63%, positive likelihood ratio 0.82, negative likelihood ratio 1.1 [Table/Fig-6].
A 2 x 2 table of urinary LAM level (cut off point 1.69 mg/dl) and results of microbiology examination.
| Urinary LAM | Microbiology examination | Total |
|---|
| Positive | Negative |
|---|
| ≥ 1.69 mg/L | 7 | 16 | 25 |
| < 1.69 mg/L | 14 | 24 | 38 |
| Total | 21 | 40 | 61 |
Using positivity of microbiology examination as reference standard, ROC curve showed AUC of this test was 0.56 (95% CI 0.41-0.72).
Discussion
Childhood TB diagnosis remains a challenge. Microbiological examination are often hampered by difficulty in obtaining samples. Moreover, diagnostic yield of sputum smear microscopy and culture is poor because TB is commonly paucy bacillary in children. Although, the reliability and validity remain unclear, scoring system and diagnostic criteria are commonly used [13,14]. Indonesia has developed and used scoring system [5] for diagnosing childhood TB at limited sources health care facilities. However, a study revealed that the scoring system had sensitivity of 47% and specificity of 68%, indicating the use of this scoring system should be re-evaluted [5].
Diagnostic performance of urinary LAM in diagnosing TB has been studied in adult population and found to have specific utility in immunocompromised patients [15]. Urinary LAM provide some advantages because samples are easy to collect compared to sputum collection which is not always doable in children. Rapid urinary LAM test result is expected to improve childhood TB management as a point of care test, since current TB diagnosis in children often delayed for days awaiting microbiology and TST result.
This study compared urinary LAM level to reference standards for both unconfirmed TB case criteria and microbiology examination (sputum microscopy and culture). These reference standards were used in accordance to case definition of pulmonary TB in children as a proposed by international expert panel [7]. This is particularly fair because microbiology examination usually has low positivity in children due to paucibacilarity of the disease [14]. Thus, the diagnostic performance would reflect the applicability in referral facilities or limited sources settings.
The study found that 43% of those diagnosed as TB were microbiologically confirmed. This slightly higher yield than that of literature [16–19] might be due to induced sputum sampling technique which can increased positive yield up to 20%. Bacteriological laboratory technique used in this study was conventional smear microscopy and culture. Indeed the newly WHO recommended Xpert MTB/RIF is twice more sensitive than smear microscopy [20], but this diagnostic tool had not been available in the hospital when the study was conducted. Otherwise, higher yield may strengthen the validity of this urinary LAM detection test [18].
Several previous studies showed that age range, malnutrition, and positive AFB and culture result could be a predictive factor related to positive urine LAM. Host and pathogen factor affected the urine LAM detection [7].
Only three subjects had HIV positive and they were actually HIV infected patients suspected and screened for TB. Expectedly, urinary LAM would be higher in these subsets [6]. Unfortunately, of those offered to have HIV screening none actually had this test performed. This makes our study can not demonstrate the effect of HIV status on diagnostic performance of urinary LAM.
LAM is heat-stable carbohydrate antigen contains glycosidic linkage. Hence, in active mycobacterial disease, LAM can be cleared through the kidney and may be detectable by sensitive technique, such as ELISA [18]. This explain the higher level of urinary LAM in subjects diagnosed with TB in the present study.
The study demonstrated good sensitivity and specificity (83.3% and 83.7, respectively) with cut off value 0.98 mg/l, and AUC 0.80 based on ROC curve. This results indicate that urinary LAM detection might be point of care diagnostic test for TB in children, especially in cases which immediate decision to treat with anti TB is needed. However, further investigation should be attempted in variable settings.
Having the microbiology examination as gold standard, urinary LAM showed poor sensitivity and specificity (33% and 60% respectively) with AUC of 0.56, which mean that this diagnostic tool is not superior to microbiological examination itself. Both LAM and microbiology examination have poor diagnostic value in childhood TB. Microbiology examination as a gold standard in diagnosing childhood TB has many limitations such as difficulty in sampling and paucibacilarity of the disease [14].
False positive result of urinary LAM may possibly caused by sample contamination with other bacteria. Anti-LAM antibody used in LAM examination can undergo cross reaction with several bacteria, including actinobacteria, candida, and non-TB mycobacteria species. Cross reaction of perineal or fecal bacteria can occur in urine contaminated from unsterile urine container. Some bacteria can grow, especially if the sample is stored at room temperature for long period and can cause bacterial replication. Therefore, clean sampling has to be done with careful instruction to subjects and their parents. The possibility of better result from middle portion urine is not clear yet, but the use of a sterile container is a must [15]. In this study, we collected random urine sample with careful precautions, such as the use of clean container to avoid false positive result.
Some studies stated that urine LAM concentration was stable in the first two hours. Urine LAM reactivity can disappear in urine sample stored for three years in the temperature of -20°C and if defrosted two times. It is suggested that the urine sample is examined immediately, or has to be frozen as soon as possible [19].
Proteinuria, haematuria, UTI, and pneumonia could affect the possibility of false positive result of urine LAM. Several other conditions could effect the results of urine LAM are UTI patients with positive culture, pneumonia with culture result of M.avium, UTI and pneumonia with both positive and negative S.pneumonia antigen. Positive result of urine LAM correlated strongly with proteinuria, indicating that urine LAM excretion possibly related to condition of renal membrane [20,21].
Limitation
The limitation of the study was urine LAM testing was performed on thawed, not fresh, and random urine samples, and it is unclear if those could impact urine LAM results. We also couldn’t evaluate the effect of HIV status on urine LAM performance, due to dissenion of HIV patients.
Conclusion
We conclude that diagnostic value of urinary LAM is good with 83.3% sensitivity and 83.7% specifity. This diagnostic test should be considered as point of care diagnostic test for childhood TB. However, further investigation are warranted to apply this test in community in adjunction to NTPs.
Data was presented in N(%), and mean±SD *HIV examination was not performed in all subjects, it was done based on parental history with HIV