Primary urinary Bladder Adenocarcinoma (PBA) is an uncommon neoplasm and can cause diagnostic difficulties due to histologic similarities with adenocarcinomas of adjacent structures like Gastrointestinal Tract (GIT) and prostate, since involvement of the bladder by metastasis or direct spread can occur.
Seven cases of bladder adenocarcinomas were diagnosed during a period of four years in a tertiary care hospital. Patient’s age ranged from 26-78 years with a male predilection. Three cases were signet ring type adenocarcinomas, two cases were subtyped as enteric variant, one as mucinous variant and one as adenocarcinoma Not Otherwise Specified (NOS) variant. One case showed urachal involvement. Common site of involvement was the base and posterior wall of the bladder. Three cases had prior history of GIT malignancy. No morphologic difference was identified to differentiate primary from secondary adenocarcinomas.
Bladder adenocarcinoma is rare tumours. Primary and secondary adenocarcinomas cannot be distinguished from each other on morphologic grounds. Ancillary studies may have limited role in distinguishing between the two. Hence, clinical correlation has a major role in their evaluation.
Urinary bladder carcinoma is the second most common tumour of the genitourinary tract. The common histopathological types include urothelial carcinoma: Transitional Cell Carcinoma (TCC) which accounts for 90% of the cases, Squamous Cell Carcinoma (SCC) and PBA accounting for 3%-7% and less than 2% respectively [1]. PBA arising from multilayered urothelium lining the bladder mucosa is fairly rare and can be broadly classified into urachal and non-urachal adenocarcinoma. It is more frequent in males in their sixth decade and common presentations include haematuria and symptoms due to bladder irritation [2]. The more commonly encountered bladder adenocarcinomas are tumours which have involved the bladder by haematogenous, lymphatic or direct spread from other organs [3,4]. These lesions have significant morphologic overlap and can cause difficulty in differentiating primary from secondary bladder adenocarcinomas.
Case Series
All patients with a diagnosis of adenocarcinoma of the urinary bladder between May, 2012 and May, 2016 were identified. Of these, seven cases for which pathology slides were available included in the study and reviewed. The clinical data and all associated ancillary investigation reports (radiology and biochemistry) were retrieved from the archives. The clinicopathologic characteristics of the seven cases are summarized in [Table/Fig-1].
Summary of the clinicopathological features.
| Case | Age/ Gender | Site | Primary elsewhere | Treatment | Follow-up | Presenting symptoms | Cystoscopy | Subtype |
|---|
| 1 | 26/ Male | Postero-superior wall | Rectum | Symptomatic | NA | Fever, UTI | NA | NOS |
| 2 | 78/ Female | Right posterolateral wall | Stomach | DJ stenting and TURBT | Contd. | Lower abdominal pain, dysuria, vomiting | Ureter-normal, average capacity of bladder decreased, bullous oedema over trigone | Signet ring type |
| 3 | 65/ Male | Base and left lateral wall | Nil | Symptomatic | NA | Loss of weight, haematuria, nocturia | External urethral meatus-normal, anterior urethra –normal, poorly distended bladder, diffuse mucosal congestion, mucosal irregularity throughout | Signet ring type |
| 4 | 52/ Male | Posterior wall and trigone | Nil | NA | NA | Haematuria, pain abdomen | NA | Signet ring type |
| 5 | 45/ Male | Right lateral wall extending till bladder neck, posterior wall with trigone | Nil | Radical cystoprostatectomy with ileal conduit | Contd. | Increased frequency, urgency, nocturia | Anterior urethra-normal, prostatic urethra-normal, vesical neck-tumour seen extending from bladder neck to base | Mucinous |
| 6 | 38/ Female | Base and right lateral wall | Rectum | TURBT | DJ stent | Pain abdomen,haematuria | 2.5 cm x 2 cm polypoidal growth seen in the area of the right ureteric orifice, right ureteric orifice could not be identified | Enteric type |
| 7 | 72/ Female | | Nil | TURBT | Diversion palliotomy | Dysuria, haematuria, abdominal pain | Meatus –adequate, urethra-congested and inflamed, urinary bladder-trabeculated and congested, papillary growth over bladder dome involving the neck | Enteric type |
NA-Not available, UTI-Urinary tract infection, TURBT-Transurethral resection of bladder tumours, NOS-Not otherwise specified
Special attention was paid to identify primary site elsewhere.
The tumours were sub classified histologically as:
Enteric type: Architecture and morphology resembles typical colon adenocarcinoma;
Mucinous: Malignant cells floating in lakes of extracellular mucin;
Signet-ring: Tumour is predominantly composed of signet-ring cells;
Adenocarcinoma-NOS variant: Tumour could not be categorized in any of the specific subtypes;
Mixed: Two or more morphological patterns, with no pattern accounting for more than 75% of the tumour [5].
Extensive search for cystitis glandularis and intestinal metaplasia, as defined by the presence of mucin secreting epithelium in nests of cystitis cystica and presence of mucin secreting epithelium in the mucosa respectively, was done [5]. Special stains like mucicarmine were done as necessary.
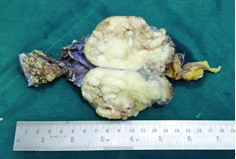
In the present case series there were four men and three women aged between 26-78 years. The most common presenting feature was haematuria and abdominal pain followed by dysuria. None of the cases had associated conditions like schistosomiasis, cystocele or bladder exostrophy. On cystoscopy, two patients showed a growth and the rest showed a combination of features like trabeculation, congestion and thickening of the bladder mucosa. One was a case of urachal adenocarcinoma [Table/Fig-2] which involved the bladder dome. Common site of involvement of the non-urachal tumours was the base and the posterior wall of the urinary bladder.
Gross appearance of the urachal tumour with a glistening white cut surface due to production of mucin.
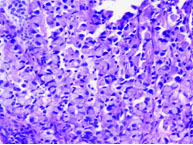
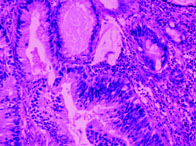
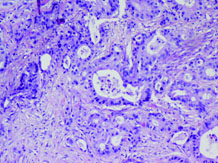
In the present case series signet ring type adenocarcinoma [Table/Fig-3] accounted for most cases (three cases) followed by enteric type (two cases) [Table/Fig-4] and one each of adenocarcinoma NOS type [Table/Fig-5] and mucinous type [Table/Fig-6]. Cystitis glandularis and intestinal metaplasia was not seen. Mucin staining with mucicarmine was done in one case which showed strong positivity [Table/Fig-7]. One case was a known case of carcinoma rectum where the bladder tumour was discovered during follow-up due to complaint of haematuria. In two cases, primary tumours were discovered in the rectum and stomach by computed tomography after histopathological diagnosis on urinary bladder. The remaining four cases were considered as primary bladder adenocarcinoma since they had no evidence of malignancy elsewhere after thorough radiological and clinical examination.
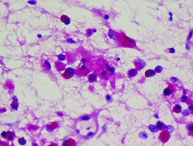
Signet ring cells containing mucin which push the nucleus to the periphery (H&E 20X).
Enteric type adenocarcinoma with architecture and morphology resembling colon adenocarcinoma (H&E 20X).
Adenocarcinoma NOS, which do not resemble any recognized type (H&E 20X).
Mucinous type with malignant cells exhibiting glandular structures (marked with arrows) floating in lakes of extracellular mucin (marked with arrow head) (H&E 20X).
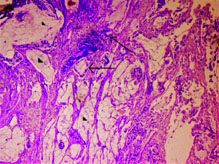
Mucicarmine staining showing strong positivity (mucicarmine 40X).
Discussion
Many of the PBA arise from the base of the urinary bladder. The male to female ratio is 3:1 in non-urachal tumours as compared to almost 1:1 in tumours arising from the urachal remnants. In our series, male to female ratio in non-urachal adenocarcinoma was 2:1. This is in concordance with other studies. The mean age in our series was 53.7 years which is similar to the results reported by Tamboli P et al., who records a younger age of fifth decade. However, Dhadania VK et al., and other authors have recorded the age of incidence to be sixth decade with a mean of around 63 years [3,6–8].
Haematuria and abdominal pain were the most common presenting complaint in our series which is consistent with the findings recorded by other workers. Additional presenting complaints include dysuria, nocturia, frequency and rarely mucusuria [4,7]. Cystoscopically, bladder adenocarcinoma does not have distinct characteristic features to reliably distinguish them from urothelial neoplasms [4].
PBA is often associated with cystitis glandularis; which is often difficult to demonstrate in small bladder biopsies and trans-urethral resection specimens. Some authors believe that PBA arise by a process of intestinal metaplasia due to chronic irritation, while others propose that PBA arise from the persisting endodermal intestinal tissue. The latter hypothesis is more befitting with urachal adenocarcinomas. Another condition which is associated with an increased incidence of PBA is ecotpia vesicae. Other risk factors include infection by schistosoma haematobium, villous adenoma and cystocele [1,2,4,8,9].
Urachal adenocarcinomas are clinically different from non-urachal tumours. They arise from the remnants which connect the bladder to the umbilicus during embryogenesis [2,4,7]. In 1954, Wheeler JD et al., had proposed that tumours of the bladder dome with the absence of cystitis cystica or glandularis, muscle invasion or infiltration to deeper structures, presence of the urachal remnant, sharp demarcation between the tumour and surface epithelium, presence of the suprapubic mass, and tumour that extends into the space of retzius can be termed urachal tumours [10]. However, the criteria were very strict and only a handful of cases met all requirements. Subsequently, Johnson DE et al., modified the criteria and proposed the following:
Tumours of the bladder dome/anterior wall;
A sharp demarcation between the tumour and the surface epithelium;
Absence of cystitis cystica/glandularis;
The exclusion of adenocarcinoma secondarily involving the bladder [8].
This modified criteria was widely accepted. However, in the absence of cystitis glandularis and intestinal metaplasia, it is a diagnostic difficulty and requires strong clinicopathological correlation. In fact Wheeler and Hill stated that “from a clinical standpoint it is justifiable to consider all adenocarcinomas of the dome as urachal, unless a transition from nonneoplastic bladder epithelium to adenocarcinoma is demonstrated” [10]. The logic being that the therapy would be same for all bladder dome tumours [11].
Various authors have opined that both these tumours have a similar pathogenesis and there is no difference between primary urachal and non-urachal adenocarcinoma in morphology and immunohistochemistry, therefore are difficult to differentiate [7,12]. However, it can be distinguished from secondary adenocarcinoma of colorectal origin which show β-catenin nuclear positivity whereas urachal adenocarcinoma shows diffuse reactivity to 34βE12. Thus, in most cases urachal adenocarcinoma is a diagnosis of exclusion after ruling out non-urachal adenocarcinomas [8].
Our case of urachal adenocarcinoma presented in the seventh decade of life. However, other authors have recorded tumours at a younger age [5,7,13]. Haematuria is the most common complaint for urachal carcinoma though mucousuria, obstructive urinary symptom and abdominal pain can be a presentation.
Urachal adenocarcinoma usually presents as a solitary polypoid mass in the bladder dome, although they may be seen anywhere along the anterior wall of the bladder. The cut surface is often glistening due to production of mucin. Enteric type with or without mucin is the common variant encountered which correlates well with our case [11]. Urachal adenocarcinomas are treated differently with en-bloc resection of bladder dome, urachal ligament and umbilicus than non-urachal tumours which are treated with radical/partial cystectomy with pelvic lymph node dissection with or without adjuvant radio/ chemotherapy. The role of adjuvant therapy is controversial as these tumours respond poorly. Some advocate radical cystectomy for all patients and the adequate treatment modality remains unsettled [1,5,7].
Patel S et al., and Grignon DJ et al., have reported adenocarcinoma NOS as the most common type of non-urachal adenocarcinoma [1,5] whereas, Roy S et al., noted the enteric type to be more common. However, in the present series, we encountered more cases of signet ring type of adenocarcinoma [2].
Common differential diagnoses for bladder adenocarcinoma include Metastatic Adenocarcinoma (MCA), urothelial neoplasm with glandular differentiation, intestinal metaplasia infiltrating the bladder wall and nephrogenic metaplasia, microcystic variant of urothelial carcinoma, cystitits cystica et glandularis [4,7].
Adenocarcinoma metastasizing to the bladder is an uncommon occurrence but PBA are relatively rarer and metastatic spread can occur from the colon, rectum, prostate, endometrium, cervix and other organs like breast, lung and kidney. Colorectal adenocarcinoma is morphologically indistinguishable from PBA. Presence of intestinal metaplasia or finger like projections may favour PBA, but overlapping features make it difficult, particularly on small biopsy specimens [7].
Immunomarkers are not very useful individually but a panel of markers may provide significant insight. A panel consisting of CK7, CK20, β-catenin and thrombomodulin has great value in differentiating MCA and PBA. Nuclear staining of β-catenin and CK20+ suggests colorectal origin whereas, CK7+, thrombomodulin+ and membranous β-catenin staining pattern suggest PBA [3,7,9]. Prostatic duct adenocarcinoma can mimic enteric type of PBA and therefore, extremely difficult to differentiate on morphology alone. Bladder infiltration by lobular carcinoma of the breast may mimic the signet ring variant of the PBA. In such situation a breast origin is favoured if immunohistochemistry (IHC) with ER, PR and GATA3 is reactive. Similar dilemma can arise in tumours when a pulmonary origin is considered and a panel comprising of Napsin A+ and TTF1+ can help minimize errors. Renal Cell Carcinoma (RCC) may involve the bladder and cause diagnostic difficulty in ruling out clear cell adenocarcinoma of the bladder. Presence of a transition from adenocarcinoma into typical urothelial carcinoma or the finding of a noninvasive urothelial component can serve as useful clue in diagnosing PBA. However, these “transitions” can be very difficult to identify and an immunohistochemistry with CD10, vimentin and RCC may prove useful [7,8,14]. Endometrial carcinoma with bladder involvement may be difficult to differentiate from PBA. Histology alone may not provide all answers. An immuno-panel comprising Ca-125, Vimentin and Pax-8 may prove useful as endometrial carcinoma show positive staining with these markers [8].
Secondaries from other organs may pose similar dilemmas. Ancillary studies with immunohistochemistry and robust clinico-radiologic correlation may go a long way in arriving at an accurate diagnosis.
Urothelium has high propensity for metaplasia with squamous metaplasia being more common than glandular, but benign glandular lesions like intestinal metaplasia infiltrating bladder wall and very florid cystitis cystica et glandularis may mimic PBA, especially on small biopsy; the lack of complex glandular architecture and cytologically atypical cells provide clue to the diagnosis. However, extensive sampling should be done to rule out any foci of conventional urothelial carcinoma or adenocarcinoma in situ [7,8]. Endometriosis in the bladder can mimic a bladder adenocarcinoma especially when the glandular proliferation is florid. Identification of endometrial stroma along with the presence of haemorrhage and/or haemosiderin laden macrophages are useful clues. In cases where cytologic atypia is prominent and obvious clues are not available, immunohistochemistry with Pax-8 serves as an important tool. Pax-8 is positive in epithelial cells of endometriosis and not in PBA. Other markers like CD10, ER and PR can also be of aid in the diagnosis in difficult situations. However, presence of this glandular differentiation may cause diagnostic dilemma. Thus, the term “bladder adenocarcinoma” which refers to tumours composed completely of glandular differentiation; an extensive search to rule out urothelial carcinoma with glandular differentiation must be made which is possible in resection specimens [7,8].
Nephrogenic metaplasia is another entity which can cause diagnostic dilemma. This usually presents as small clusters of tubular structure or glands which may resemble renal tubules. The cells lining these structures may be cuboidal to columnar and may have cytologic atypia or cytoplasmic vacuoles resembling signet ring like cells. However, presence of an oedematous stroma with inflammatory infiltrate and thick basement membrane seen in haematoxylin and eosin stained sections favours a diagnosis of nephrogenic metaplasia. Further, it does not show large amount of mucin, mitosis, necrosis and diffuse cytologic atypia [8].
Conclusion
PBA is an uncommon bladder tumour. Various morphologic patterns have been described. The possibility of metastasis from other sites has to be ruled out before a diagnosis of PBA is rendered. The clinic-radiological correlation and ancillary studies are very useful in this scenario. Urachal adenocarcinoma is a distinct group of adenocarcinomas diagnosed on the basis of strict diagnostic criteria.
NA-Not available, UTI-Urinary tract infection, TURBT-Transurethral resection of bladder tumours, NOS-Not otherwise specified
[1]. Patel S, Rathod G, Shinde P, Tandan RK, Adenocarcinoma of urinary bladder in 55-year-old male patient-A rare case reportIAIM 2015 2(1):116-20. [Google Scholar]
[2]. Roy S, Smith MA, Cieply KM, Acquafondata MB, Parwani AV, Primary bladder adenocarcinoma versus metastatic colorectal adenocarcinoma: a persisting diagnostic challengeDiagnostic Pathology 2012 7:151 [Google Scholar]
[3]. Tamboli P, Mohsin SK, Hailemariam S, Amin MB, Colonic adenocarcinoma metastatic to the urinary tract versus primary tumours of the urinary tract with glandular differentiation a report of 7 cases and investigation using a limited immunohistochemical panelArch Pathol Lab Med 2002 126:1057-63. [Google Scholar]
[4]. Sigalas K, Tyritzis SI, Trigka E, Katafigiotis I, Kavantzas N, Stravodimos KG, A male presenting with a primary mucinous bladder carcinoma: a case reportCases Journal 2010 3:49 [Google Scholar]
[5]. Grignon DJ, Ro JY, Ayala AG, Johnson DE, Ordonez NG, Primary adenocarcinoma of the urinary bladder a clinicopathologic analysis of 72 casesCancer 1991 67:2165-72. [Google Scholar]
[6]. Roy S, Parwani AV, Adenocarcinoma of the urinary bladderArch Pathol Lab Med 2011 135:1601-05. [Google Scholar]
[7]. Dadhania VK, Czerniak B, Guo CC, Adenocarcinoma of the urinary bladderAm J Clin Exp Urol 2015 3(2):51-63. [Google Scholar]
[8]. Jhonson DE, Hodge GB, Abdul-Karim FW, Agala AG, Urachal carcinomaUrology 1985 26(3):218-21. [Google Scholar]
[9]. Broede A, Oll M, Maurer A, Siegert S, Stoerkel S, Golz R, Differential diagnosis of bladder versus colorectal adenocarcinoma: keratin 7 and GATA3 positivity in nuclear ß-catenin-negative glandular tumours defines adenocarcinoma of the bladderJ Clin Pathol 2016 69:307-12. [Google Scholar]
[10]. Wheeler JD, Hill WT, Adenocarcinoma involving the urinary bladderCancer 1994 7:119-35. [Google Scholar]
[11]. Gopalan A, Sharp DS, Fine SW, Tickoo SK, Herr HW, Reuter VE, Urachal carcinoma: A clinicopathologic analysis of 24 cases with outcome correlationAm J Surg Pathol 2009 33(5):659-68. [Google Scholar]
[12]. Anderstrom C, Johansson SL, Schultz LV, Primary adenocarcinoma of the urinary bladder -a clinicopathologic and prognostic studyCancer 1983 52:1273-80. [Google Scholar]
[13]. Valerio M, Lhermitte B, Bauer J, Jichilnski P, Metastatic primary adenocarcinoma of the bladder in a twenty-five years old womanRare Tumours 2011 3(9):28-29. [Google Scholar]
[14]. Mukhopadhyay S, Katzensteinn AL, Comparison of monoclonal Napsin A, polyclonal Napsin A, and TTF-1 for determining lung origin in metastatic adenocarcinomasAm J Clin Pathol 2012 138:703-11. [Google Scholar]