Fine Needle Aspiration Cytology (FNAC) has become an important pre-operative and screening test for various lesions. It is also a valuable aid for screening of malignant and potentially malignant lesions. It is easy, economical, non-invasive, quick and feasible method for detection of malignancies [1–3]. The Papanicolaou (PAP) stain was developed by Dr. George N Papanicolaou, the Father of Cytopathology. It is a multi-chromatic staining technique developed in 1942 and subsequently modified in 1954 and 1960 [4]. PAP stain is used to identify and differentiate cells in smears prepared from various body fluids, gynaecological smears and FNAC smears from various organs [1,2]. Diagnosis by FNAC has a major role in good and efficient medical practice. It is easy to perform and can be done quickly. The need of the hour is minimal turnaround time and quick diagnosis on FNAC. This has encouraged many newer staining techniques with lesser staining time with unequivocal cell morphology [2,3].
The ever increasing use of FNAC as one of the pivotal diagnostic tools has validated the use of other stains like Romanowsky and Haematoxylin and Eosin (H&E) stains along with the conventional PAP stain [4]. Few rapid stains are available now-a-days which include Diff-Quick stain, Toluidine blue stain and May Grunwald Giemsa (MGG) stain. However, most of the cytopathologists prefer a multichromatic, transparent stain with crisp nuclear and cytological characteristics which are offered by wet 95% ethanol fixed PAP stain and not by air dried smears stained by Romanowsky stain [3,5].
Kamal MM et al., invented the MUFP stain as reagents required for the UFP stain were not universally available [7]. They used easily available Gills or Harris haematoxylin instead of Richard Allan haematoxylin and modified Eosin Azure (EA) instead of Richard Allan cytostain.
Hence, the present study was undertaken to assess the efficacy of MUFP staining in cytological diagnosis of various lesions in the body and comparing these results with conventional PAP stain.
Materials and Methods
The present study included 131 cases of FNAC which was carried out from first November 2013 till 31st July 2015 over a period of one year and nine months in the Department of Pathology, BLDE University, Shri BM Patil Medical College, Vijayapur, Karnataka, India. Patients referred for FNA of various lesions sent to the cytology section of the Department of Pathology for cytological evaluation were included. FNAC procedure was performed in our laboratory by using Cameco syringe pistol with 10 ml disposable syringe and 22-23 G needle and multiple smears were prepared. A total of two smears were then selected in our study on clean glass slides of which one smear was fixed in 95% ethanol for minimum 15 minutes. This smear was stained with conventional PAP stain and the other smear was air dried and rehydrated with normal saline and was subsequently fixed in alcoholic formalin and stained by MUFP stain.
Inclusion Criteria
All patients referred for FNAC to the Department of Pathology for cytological evaluation during the study period.
Exclusion Criteria
FNAC smears with inadequate material and only haemorrhagic material were excluded.
Staining Procedure of MUFP [
8]
The smears were air dried for about 30 minutes and then flooded with 0.9% normal saline for 30 seconds followed by alcoholic formalin for about 10 seconds. This was the fixation procedure which was followed by the staining technique.
Staining was done by dipping the smears in tap water followed by Harris haematoxylin for 30 seconds and then tap water again six slow dips. This was then followed by ethyl alcohol 95% six dips and EA 36 for 15 seconds. Again ethyl alcohol 95% followed by 100% ethyl alcohol each six dips were done. Finally 10 slow dips in xylene were done followed by mounting of the slides.
Staining quality was assessed for parameters like smear-background, overall staining, nuclear character, and cell morphology as shown in [Table/Fig-1].
Scoring used for both stains.
| Parameter | Score |
|---|
| Background | Clean | 2 |
| Haemorrhagic | 1 |
| Overall Staining | Good | 3 |
| Moderate | 2 |
| Bad | 1 |
| Cell Morphology | Well preserved | 3 |
| Moderately preserved | 2 |
| Poorly preserved | 1 |
| Nuclear Character | Good | 3 |
| Moderate | 2 |
| Poor | 1 |
The maximum score for each case, taking into account all the 4 parameters, was 11. The QI
{QI = actual score obtained /maximum score possible} for each case with both the stains was then obtained by calculating the ratio of actual score to the maximum score possible.
Statistical Analysis
Statistical analysis using chi square test was applied to each of the four parameters before obtaining the QI in both stains.
Before calculating the mean, statistics was applied first to specific organs and then inter organ comparison was calculated. Students t-test was applied to evaluate the two independent means with a significance level of 0.10 and a two tailed hypothesis.
Diagrammatic representation of the data.
Results
Total 131 cases were studied and catergorized organ wise into lymph node (30), thyroid (38), breast (22), skin and soft tissue (24), salivary gland (11) and visceral organs (6). Each organ was evaluated for various lesions separately and comparison between the two stains was then done.
From [Table/Fig-2] we concluded that a clean background was seen in 91.6% cases of MUFP stain as compared to only 66.41% cases of PAP stain making the difference statistically significant [Table/Fig-3,4]. Overall staining was good in 49.6% of MUFP stain as compared to 31.30% on PAP stain [Table/Fig-5,6]. Poor staining was seen in 4.58% cases of PAP stain making the difference statistically significant. Cell morphology was well preserved in both cases of MUF and PAP stain making the statistical difference insignificant [Table/Fig-4,6,7]. Also, prominent cytoplasmic keratinization was well appreciated with PAP stain as compared to MUFP stain where orangiophilia was lost [Table/Fig-8]. This helped in better and definitive diagnosis of squamous cell carcinoma cases in all organs. Nuclear characteristics were better on PAP stain being 80.92% as compared to only 42.75% on MUFP stain [Table/Fig-6,9], again making the difference statistically significant.
Thyroid follicular cells: a) Haemorrhagic background, PAP X400; b) Clean background, MUFP X400.
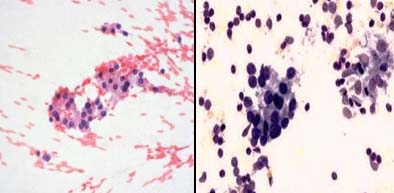
Fibroadenoma breast: a) With bare bipolar nucleai, PAP X200; b) With crisp differential staining, MUFP X200.
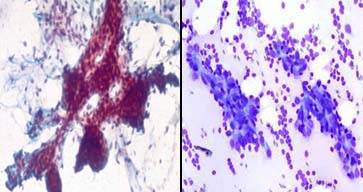
Intraductal carcinoma breast; a) With poor overall staining, PAP X600; b) With good overall staining with better cell morphology MUFP X600.
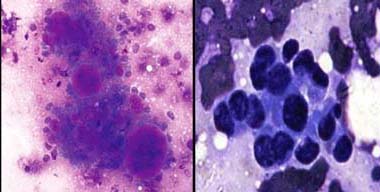
Non hodgkins lymphoma: a) Showing moderate to poor staining, PAP X 400; b) Better overall staining with clearer cell morphology MUFP X400.
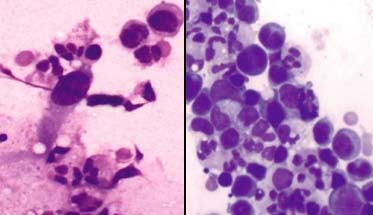
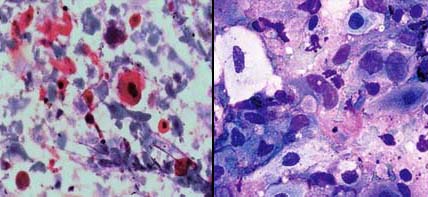
Hodgkins disease; a) Showing prominent RS cells with crisp nuclear features, PAP X400; b) Nuclear features are indistinct, MUFP X400.
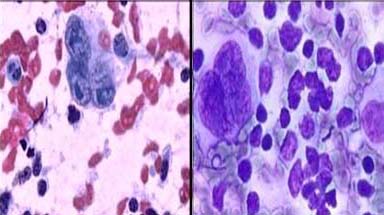
Metastatic adenocarcinoma: a) With haemorrhagic background showing obscured acinar patter, PAP X100; b) Prominent acinar pattern on clean background, MUFP X100.
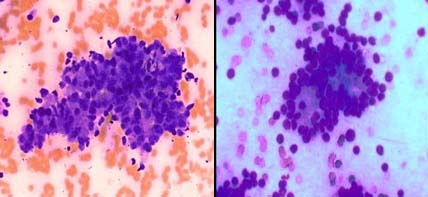
Metastatic squamous cell carcinoma: a) With prominent cytoplasmic keratinization, PAP X400; b) Orangiophilia is lost, MUFP X400.
Comparison of the parameters on both stains (131 cases).
| STAINS | MUFP | PAP |
|---|
| Background |
| Clean | 120 (91.60%) | 87 (66.41%) |
| Haemorrhagic | 11 (8.40%) | 44 (33.59%) |
| Overall Staining |
| Good | 65 (49.62%) | 41 (31.30%) |
| Moderate | 65 (49.62%) | 84 (64.12%) |
| Bad | 1 (0.76%) | 6 (4.58%) |
| Cell Morphology |
| Well preserved | 95 (72.52%) | 98 (74.81%) |
| Moderately preserved | 36 (27.48%) | 32 (24.43%) |
| Poorly preserved | 0 | 1 (0.76%) |
| Nuclear Characteristics |
| Good | 56 (42.75%) | 106 (80.92%) |
| Moderate | 74 (56.49%) | 25 (19.08%) |
| Bad | 1 (0.76%) | 0 |
QI was calculated for all the slides of each organ and then mean of these was further calculated.
The results were as follows:
Thus, from the results in [Table/Fig-10,11] it was concluded that in thyroid MUFP stain had a better QI than PAP stain. In breast samples both stains were equally good in respect to their QI. In lymph node and salivary gland MUFP stain had a better QI than PAP stain while in cases of visceral organs PAP stain had a better QI than that of MUFP stain.
| ORGANS | QI of MUFP | QI of PAP |
|---|
| Thyroid | 0.889 | 0.81 |
| Breast | 0.85 | 0.854 |
| Lymph node | 0.883 | 0.803 |
| Skin and soft tissue | 0.825 | 0.833 |
| Salivary gland | 0.918 | 0.854 |
| Visceral organs | 0.788 | 0.811 |
Students t-test and p-value with significance level of 0.10.
| t value | p-value | Result |
|---|
| 1.458373 | 0.08771 (p < 0.10) | Significant |
Discussion
In the present study, we compared the cytomorphological characteristics of rehydrated air dried smears stained by MUFP stain with ethyl alcohol fixed smears stained by conventional PAP stain.
Shinde PB et al., observed that with MUFP staining complete air drying revealed a clean, RBC free background [8]. Kamal MM et al., also observed that the problem of air drying artefacts in wet fixed smears can be reduced by rehydration of air dried smears as in MUFP [9]. Maruta J et al., showed that MUFP stain makes the background haemorrhage free by lysing, the RBCs [10]. This also makes the smear thinner and clearer for cytomorphologic observation.
In our study, [Table/Fig-2] in MUFP stain clean background was seen in 91.6% cases as compared to 66.4% cases in wet fixed smears PAP stain. The p-value was calculated by using student t-test which proved the difference to be statistically significant [Table/Fig-11]. These findings are comparable to findings of studies done by Shinde PB et al., Kamal MM et al., Maruta J et al., and Patrikar A et al., [8–11]. Thus, our study showed that air-dried smears rehydrated within 30 minutes with normal saline for about 30 seconds provide cleaner and RBC free background compared to the wet fixed PAP smears.
Also, the timing of rehydration after complete air drying was very important and is highlighted in various studies. It was observed that for optimal cell preservation and complete haemolysis, it was best if air dried smears were rehydrated within 30 minutes for 30 seconds. An optimum timing of 30 seconds in normal saline was quoted in studies done by, Shinde PB et al., Kamal MM et al., and Maruta J et al., [8–10]. This timing was strictly observed in our study and we got good results.
The rehydration technique, discovered by Chang and Kung using normal saline restores the transparency of air-dried cells [12]. In addition it haemolysis the RBC’s in the smear and unmasks tumour cells. Cells were larger because of air-drying, nucleoli were distinctly seen and stain red. Thus, our study shows that air-dried smears rehydrated with normal saline show cleaner, non-haemorrhagic background as compared to only air-dried smears and only wet fixed smears.
Yang GC and Alvarez II advised use of fresh saline for every case in order to avoid incomplete haemolysis of background red blood cells [6]. Kamal MM et al., optimized the change of normal saline after every five smears [9]. We followed use of fresh normal saline in every case.
Yang GC and Alvarez II and Kamal MM et al., advised daily changing of alcoholic formalin [6,9]. This solution is storage sensitive and a pH of 5 should be maintained. Prolonged immersion in this fixative affected cytomorphology and caused blurring and wrinkling of nuclei. In our study, we followed daily changing of alcoholic formalin and maintained it at a pH of 5.
Shinde PB et al., and Kamal MM et al., observed that delay in changing haematoxylin caused faint staining of nuclei and thus, affected morphology [8,9]. They advised change of haematoxylin after 60 smears. We changed the solutions after every 30 smears and hence, got good morphology and results.
Shinde PB et al., and Kamal MM et al., advised change of EA after processing of 60 smears [8,9].
The cytoplasmic stain used in our study was an alcoholic mixture of eosin Y, light green, phosphotungstic acid and glacial acetic acid. This was similar to that used by Kamal MM et al., Shinde PB et al., Patrikar A et al., and Choudhary P et al., in their study [8,9,11,13]. Yang GC and Alvarez II and Yang GCH and Hoda SA used Richard-Allan cytostain which was an alcoholic mixture of orange-G, eosin Y, light green and aniline blue [6,14]. As there was lack of orange- G component in the cytostain of our study, it produced difficulties in interpretation of smears of squamous cell carcinoma. We observed that, categorization of metastatic squamous cell carcinoma in lymph node cases was not confirmatory due to lack of cytoplasmic keratinization of the squamous cells. A similar problem for diagnosis of squamous cell carcinoma was noted by Shinde PB et al., Kamal MM et al., Patrikar A et al., and Choudhary P et al., in their studies [8,9,11,13].
From the above, [Table/Fig-12] we saw that Shinde MM et al., calculated QI of four organs that is in lymph node, thyroid, breast, and salivary gland [8]. QI in their study was 0.98 for thyroid and lymph node, 0.92 for breast and 0.95 for salivary gland. However, in our study [Table/Fig-12] it was 0.89 for thyroid and lymph node. This may be because the number of cases in their study was much lesser which led to a high QI.
QI of different organs in various studies for MUFP.
| Studies | Shinde PBet al [8] | Patrikar A et al [11] | Choudhary P et al [13] | Present Study |
|---|
| Organs | No. of Cases | QI | No. of Cases | QI | No. of Cases | QI | No. of Cases | QI |
|---|
| Thyroid | 7 | 0.98 | 8 | 1 | 25 | 1 | 38 | 0.89 |
| Breast | 16 | 0.92 | 12 | 0.9 | 23 | 0.97 | 22 | 0.85 |
| Lymph node | 15 | 0.98 | 11 | 1 | 43 | 0.98 | 30 | 0.89 |
| Salivary gland | 2 | 0.95 | - | - | - | - | 11 | 0.92 |
| Soft tissue | - | - | 3 | 1 | 9 | 1 | 24 | 0.83 |
Choudhary P et al., also calculated QI scores of MUFP in four organs and compared with rapid PAP stain [13] [Table/Fig-12]. They found that MUFP smears have RBC free and clean background. This helped them greatly in interpretation of thyroid lesions giving a QI of one for thyroid lesions.
QI in breast lesions was slightly lower in studies by Shinde PB et al., and Choudhary P et al., [8,13] [Table/Fig-12]. They explained that the index was lower due to sub-optimal staining of single bipolar nuclei in the background in cases of fibroadenoma. However, myoepithelial cells in the clusters were well stained. Hence, it was possible to make a diagnosis of benign breast lesion. They were unable to explain the precise reason as to why the bipolar nuclei in the background were stained suboptimally. The stromal nature of these cells may be responsible for inadequate staining. Similar explanation may be the reason for low QI in breast lesions in our study. In our study, out of the 22 cases of breast lesions, 10 cases were that of fibroadenoma, hence, QI may be even lower.
Inability to obtain optimum staining even in the pleomorphic adenoma of salivary gland has been observed but well documented studies are not available. Kamal MM et al., claim that this could be a result of poor penetration of the cytostain and these modifications needs to be addressed in further studies [9]. In our study, out of the 11 cases of salivary gland lesions, only two cases were of pleomorphic adenoma and hence, we obtained a comparatively better QI for this group.
MUFP stain gives a clean RBC-free background because of rehydration of air-dried smears by normal saline [11]. A better interpretation is possible, as the epithelial cells are not obscured by RBCs. Also, MUFP stain heightens the quality of nuclear characteristics, essential for cytological diagnosis.
Hence, it is useful for rapid diagnosis except in squamous cell lesions [8,9,12,14].
Limitation
MUFP stain is technique sensitive. Complete air drying needs to be strictly observed. Inadequate drying gives suboptimal results. Alcoholic formalin is storage sensitive and the pH of alcoholic formalin should be maintained at 5.0 or it can lead to poor staining. Normal saline, Harris haematoxylin and EA-36 need to be changed regularly. The p value between 0.05 to 0.1 shows a very weak evidence that the null hypothesis does not hold. So, a study on a large sample size must be conducted to validate the results further.
Conclusion
FNAC is a rapid, safe and cost effective technique for on-site cytological diagnosis. MUFP is a rapid stain, with staining time of 130 seconds and easily available reagents in Indian set up. MUFP smears showed cleaner background. Air drying artifacts are less in rehydrated MUFP smears as compared to wet fixed PAP smears. Since orange G gives a dirty orange background, it is omitted from MUFP stain. As a result, the interpretation of cytoplasmic keratinization is not possible. Nuclear crispness and prominent nucleolar features are better appreciated on conventional PAP stain as compared to MUFP stain.
Thus MUFP is fast, reliable and can be done with locally available reagents with unequivocal morphology which is the need of hour for a cytopathological set-up concluding that MUFP stain can be used as routine cytological stain.