Tuberculosis (TB), being a disease known to mankind since many centuries, continues to be a public health menace, especially in the underdeveloped countries. On the other hand, global incidence of Chronic Obstructive Pulmonary Disease (COPD) and other obstructive airway diseases are gradually increasing owing to increased risk factors of airflow limitation. According to the global burden of disease and risk factors, COPD and TB are among the top ten causes of death and disability for underdeveloped countries at the beginning of the 21st century [1].
Several previous studies suggested an association between Pulmonary Tuberculosis (PTB) and airflow limitation, which were conducted in Africa [2–4], and in Asia [5–8]. A South African study, which was done on subjects who had previously been treated for PTB up to sixteen years ago, also showed that 68% of the patients had chronic obstructive lung disease [9]. Thereby, it can be said that patients surviving PTB are left with permanent damages in lung architecture and are at higher risk of pulmonary sequelae. Although TB has also been mentioned as a risk factor of COPD in recent Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline [10], current definition of COPD does not include infectious aetiology. Thereby, we preferred the “Obstructive Airway Disease (OAD)” terminology over COPD in our study.
Materials and Methods
It was a cross-sectional, observational and comparative study, carried out in the pulmonary medicine OPD of V.S.S. Medical College, Burla, India, between October 2014 and September 2015. The procedures followed in this study were in accordance with the ethical standards of our Institutional Committee on human experimentation.
All adult (≥ 18 years) patients with past history of adequately treated PTB (based on history given by the patient and review of old document, whenever available), who presented to the OPD with respiratory complaints, were consecutively selected for the study. Patients with evidence of active TB (on the basis of two sputum smear for acid-fast bacilli and chest X-ray) and inadequately treated previous TB (e.g., wrong antituberculous drug regimen, inadequate dosage, incomplete course of therapy) were excluded from the study. Subjects with history of OAD or other chronic lung disease prior to TB were also excluded. HIV-positive patients were left out as HIV itself is an independent risk factor for COPD [10].
Patients who fulfilled our selection criteria were interviewed with a structured questionnaire regarding present complaints with duration, personal history (occupation, smoking etc.,). Detail of past TB and its therapy was recorded according to patient’s history and old documents, whenever available. “Smoker” was defined according to Centres for Disease Control and prevention (CDC), USA guideline as the individuals who smoked at least 100 cigarettes or its equivalent in their lifetime [13]. Extent of smoking was subsequently assessed using ‘Smoking Index’ (SI) i.e., number of bidi or cigarette smoked per day multiplied by duration of smoking in years. Smokers were categorized as mild smoker (SI<100), moderate smoker (SI=100-300) and heavy smoker (SI>300) [14]. General survey, vital signs including saturation of oxygen measured by pulse oximetry (SpO2) and detailed respiratory system examination findings were documented. Chest X-ray (Postero-anterior view) was done and evaluated for signs of inactive TB. Extent of lesions (pulmonary fibrosis, cavity, parenchymal calcification and bronchiectasis) in chest X-ray was assessed by two clinicians according to Willcox classification [9]. Each lung was divided into three zones in this classification and extent was described as follows:
Degree I – Minimum involvement in only one zone, without cavitation.
Degree II – Involvement of two/ three zones with/ without cavitation or involvement of one zone with cavitation.
Degree III – Severe involvement involving more than three zones with or without cavitation.
However, normal chest X-ray, pleural thickening or calcification, hyperinflation of lung or any other abnormality was categorized as “Others”.
Subsequently spirometric evaluation was done by a portable spirometer, SPIROLAB II {manufactured by Medical International Research, Italy; and meets American Thoracic Society and European Respiratory Society standards (ATS & ERS)}, before and fifteen minutes after administration of 400 microgram salbutamol using pressurized metered-dose inhaler (pMDI) with small-volume spacer device. All patients were instructed not to use any bronchodilator on the preceding night and on day of procedure. Spirometric procedure was carried out as per ATS/ERS task force recommendation for standardization of lung function testing [15]. Subjects who were found to have post-bronchodilator FEV1 (Forced Expiratory Volume in first second)/FVC (Forced Vital Capacity) <0.7 were taken up for final analysis as this value indicates the cut-off for diagnosis of OAD according to GOLD guideline. Bronchodilator Reversibility (BDR) was defined as an improvement in FEV1 by at least 12% and 200 ml over pre-bronchodilator value. FEV1/FVC ≥0.7 were excluded as those patients had either a normal spirometry or a purely restrictive ventilatory abnormality. Also, the individuals who failed to fulfil acceptability and reproducibility criteria of spirometry were excluded.
FEV1, FVC, PEFR (Peak Expiratory Flow Rate) and FEF 25-75 (Mid-expiratory flow rate) were recorded for finally selected patients. Study population was divided into two groups: purely obstructive abnormality and mixed ventilatory abnormality. Purely obstructive disorder group was defined as population with FVC ≥80% whereas, OAD patients with associated FVC <80% were classified as mixed ventilatory abnormality group. Spirometric parameters were compared between these two groups. Severity of airflow limitation was assessed using post-bronchodilator FEV1 based on latest GOLD guideline (i.e., FEV1≥80% as mild, 80%>FEV1≥50% as moderate, 50%>FEV1≥30% as severe and FEV1<30% as very severe) [10]. Spirometric parameters were also compared between smoker and non-smoker group to find out whether smoking alters the severity of airflow limitation in this study population.
Statistical Analysis
Data were compared for statistical significance using Fisher’s-test, Chi-square test and Student’s t-test, as appropriate. All analyses were performed using the IBM SPSS statistics for Windows, version 24.0 (IBM Corp., Armonk, N.Y., USA).
Results
A total of 274 adult patients (190 male and 84 female) with prior history of PTB attended OPD with respiratory complaints within the study period. Thirty patients were excluded as they had active TB. Twenty-six more patients were excluded based on our exclusion criteria (6 had HIV, 14 had prior history of inadequately treated TB, 6 had chronic respiratory disease prior to having TB). Spirometric evaluation was done in 218 patients and out of them, 20 were excluded as their spirometry did not meet acceptability and reproducibility criteria even after repeated attempt. Twenty-two patients were found to have normal spirometric values and 38 had purely restrictive impairment [Table/Fig-1].
Flow diagram of the study sample.
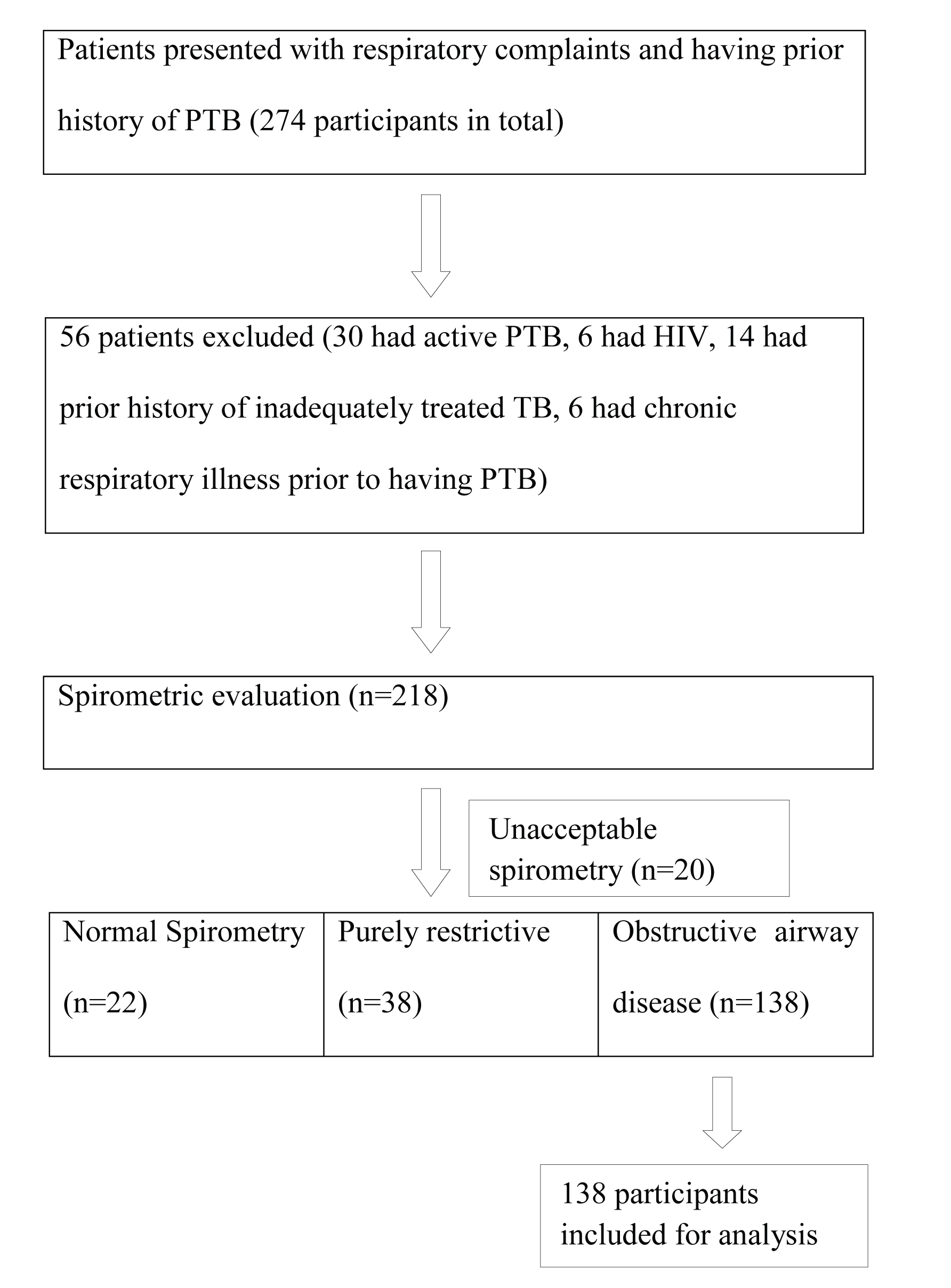
Therefore, a total of 138 patients were selected for final analysis. Detail of the baseline characteristics of the study population has been provided in [Table/Fig-2]. Mean age of the study population was 53.39±13.86 years. There were 60 smokers (43.48%) and 78 non-smokers in our study group. Among those 60 smokers, eight were mild smoker, 10 were moderate smoker and rest 42 were heavy smoker. A total of 33.33% of our study population (46 of 138) were farmer by occupation. Dyspnoea was found out to be the most common symptom (in 95.65% cases) followed by cough (89.85%) and expectoration (75.36%). A total of 74 of 138 patients (53.62%) developed current respiratory symptoms more than six years after the completion of their antituberculous treatment.
Age and sex distribution of the study population.
| Age Group (in years) | Male | Female | Total |
|---|
| 20-29 | 4 (2.89%) | 4 (2.89%) | 8 (5.79%) |
| 30-39 | 16 (11.59%) | 2 (1.45%) | 18 (13.04%) |
| 40-49 | 16 (11.59%) | 6 (4.35%) | 22 (15.94%) |
| 50-59 | 32 (23.19%) | 2 (1.45%) | 34 (24.64%) |
| 60-69 | 34 (24.64%) | 8 (5.79%) | 42 (30.43%) |
| 70-79 | 14 (10.14%) | 0 (0%) | 14 (10.14%) |
| Total | 116 (84.06%) | 22 (15.94%) | 138 (100%) |
According to Willcox classification, Degree II was the most common radiological extent of inactive TB found in 72 out of 138 (i.e., 52.17%), followed by Degree I (24.64%) and Degree III (17.39%). Severity of airflow limitation was found to be dependent on extent of radiological lesion and this relationship was statistically significant (p<0.001, Chi-square test). It means greater degree of radiological lesion is associated with more severe airflow obstruction [Table/Fig-3]. It should also be noted that all the patients with radiological lesion of Degree III (n=24) had mixed ventilatory pattern on spirometry. On the other hand, out of all patients with pure obstruction (n=38), two had only hyperinflated lung without any fibrosis, 12 had Degree I and 24 had Degree II radiological lesion.
Comparison of extent of radiological lesion with staging of obstructive airway disease.
| CXR* Lesion Extent | GOLD I(Mild) | GOLD II(Moderate) | GOLD III(Severe) | GOLD IV(Very Severe) | Total |
|---|
| Degree I | 2 | 20 | 8 | 4 | 34 |
| Degree II | 8 | 30 | 22 | 12 | 72 |
| Degree III | 0 | 0 | 10 | 14 | 24 |
| Others | 2 | 2 | 4 | 0 | 8 |
| Total | 12 | 52 | 44 | 30 | 138 |
*CXR: Chest X-Ray
Hundred OAD patients had additional restrictive component as per the spirometric results and 88% of them showed poor response to short-acting bronchodilator. On the other hand, BDR was significantly greater in purely obstructive group and 22 out of 38 patients had good response to salbutamol inhalation (p<0.001, Chi-square test). Regarding post-bronchodilator FEV1 (in litre) and FEV1% predicted values, patients with mixed ventilatory abnormality had significantly worse values than patients with purely obstructive disorder (p<0.001, Student’s t-test) [Table/Fig-4].
Comparison of FEV1 between patients with mixed pattern and purely obstructive pattern.
| Post-Bronchodilator | Mixed (n=100) | Obstructive (n=38) | p-value |
|---|
| FEV1 (L) | 0.996±0.328 | 1.475±0.499 | <0.001 |
| FEV1 % predicted | 42.28±14.54 | 67.79±15.74 | <0.001 |
Smokers had a mean difference of 8.08±5.70 years between completion of anti-tuberculous treatment and onset of present symptoms of OAD whereas, the difference was 9.18±5.33 years in non-smokers. Although smokers apparently developed symptoms of OAD one year earlier than non-smokers, but it was not statistically significant (p=0.24). No significant difference was noted in their mean SpO2 value as well (92.63±3.123 vs 92.05±3.531; p=0.31 by unpaired t-test). Post-bronchodilator percentage predicted values of FVC, FEV1, PEFR and FEF25-75 were compared between smokers and non-smokers [Table/Fig-5], but no significant difference was found for any of those parameters. We could not even demonstrate any significant difference in post-bronchodilator FEV1 among mild, moderate and heavy smokers.
Comparison of post-bronchodilator spirometric parameters among smokers and non-smokers.
| Spirometric Parameters | Smokers (n=60) | Non-smokers (n=78) | p-value |
|---|
| FVC% predicted | 72.27±20.730 | 67.72±24.117 | 0.245 |
| FEV1% predicted | 53.24±21.107 | 48.43±17.335 | 0.144 |
| PEF% predicted | 39.53±15.645 | 36.22±15.589 | 0.219 |
| FEF 25-75% predicted | 23.64±15.831 | 20.37±13.178 | 0.187 |
Discussion
Although post-TB OAD is a well-documented entity since many years, TB patients are not routinely counselled or followed up for post-tuberculous sequelae. Often such patients are wrongly treated for TB on multiple occasions on the basis of chronic cough, expectoration or haemoptysis. In the present study, we evaluated the clinical and spirometric profile of such post-TB OAD patients.
The exact pathogenesis of post-TB OAD is not clear but an immunological hypothesis has been put forward. It has also been postulated that TB causes destruction of lung parenchyma by up-regulation of different proteases and dysregulation of protease control mechanism [16]. Pulmonary damage by matrix metalloproteinase leads to cicatricial transformation of lung tissue and such fibrotic changes are probably responsible for associated restrictive disorder. Verma SK et al., in their study at Lucknow found that incidence of dyspnoea was the most common within five years of completion of anti-tuberculous treatment [8], and Rajasekaran S et al., described development of post-TB asthma within one year of completion of therapy in 50.9% of their 55 patients [17]. On the other hand, Krishna K et al., mentioned that obstructive changes were the most common after ten years of follow up [18]. Similar finding has been described by Baig IM et al., in their study among 47 post-PTB patients with chronic dyspnoea at Rawalpindi, Pakistan [19]. In our study population, more than half of the patients developed features of OAD for the first time after six years since completion of anti-TB therapy; however, possibility of recall bias cannot be excluded owing to chronicity of the OAD symptoms. As OAD can develop anytime during the course of PTB or even after successful completion of antituberculous therapy, it can be logically said that prevalence of OAD increases with number of years passed since successful treatment of TB [20].
We observed predominantly mixed pattern of spirometric abnormality in the study population and such fact may be supported by results of several other studies [21–23]. Such a high prevalence of mixed pattern can be explained by the extensive fibrosis co-existing with airflow obstruction. This sort of fibrotic sequelae is quite common in India as people often present to health facility late in course of their TB owing to poor socio-economic condition. Verma SK et al., described similar findings in their study among 92 post-PTB individuals and they found restrictive pathology in 37 and mixed pattern in 21 patients as per spirometric criteria [8]. However, 55.3% of post-PTB chronic dyspnoea cases (n=47) had obstructive ventilatory disorder without any restrictive component in above mentioned Rawalpindi study [19]. Also, most of the patients with mixed spirometric pattern in our study showed poor BDR which may indicate that cicatricial fibrosis is a hindrance to bronchodilatory effect of salbutamol. But, Verma SK et al., did not describe any significant difference in BDR between purely obstructive and mixed disorder group [8].
It had been documented that PTB occurred more commonly among smokers since long. Probably this is due to the fact that nicotine turns off Tumour Necrosis Factor-alpha (TNF-α) production by the lung macrophages, thereby making the patient more susceptible to the development of progressive disease from latent Mycobacteriumtuberculosis infection [24]. But regarding post-TB OAD, present study failed to establish any relation between smoking and severity of airflow limitation. Similar finding was also described by Ramos LM et al., Di Naso et al., Singla et al., and Lam KB cohort study [21–23,25]. A strong association between a medical history of TB and airflow obstruction has been proved beyond doubt by a large, population based, multicentre study in Latin America (PLATINO study) [26]. However, no definite hypothesis has been put forward to explain such findings in above mentioned studies. Possibly, in country like India, people are exposed to many risk factors for COPD apart from smoking; like indoor or outdoor air pollution, occupational exposure to dust/smoke which might have played a role of confounding factors among non-smoker patients in this study, thereby diluting the overall effect of tobacco smoking.
Limitation
Present study involved only one tertiary care hospital and had a relatively small study sample. We have included only symptomatic patients in our study. Thereby all asymptomatic patients were left out who might have spirometric features of pulmonary function impairment. In this study, “smoker” group included both current and ex-smokers. Presence of subjects who stopped smoking several years ago in the “smoker” group might be a confounding factor in comparison between smoker and non-smoker group.
Post-TB patients with normal spirometry or only restrictive impairment were not evaluated in our study. So, the actual scenario of post-TB pulmonary impairment could not be assessed. Also, total lung volume estimation by body plethysmography was not done due to lack of facility. Therefore, it could not be confirmed whether some patients with severe obstructive disorder had been misclassified as mixed spirometric abnormality.
Conclusion
Such pulmonary function impairment after treatment of TB is still a less recognized cause of chronic lung disease worldwide. Physicians should be aware of the long-term risk of developing airflow limitation in individuals with prior TB, irrespective of smoking status. Such patients commonly have additional restrictive ventilatory disorder, which further complicates the issue. Proper counselling to PTB patients and regular follow up even after completion of anti-TB therapy is absolutely essential to detect post-TB OAD at its earliest as it may develop anytime during or after TB treatment. Periodic spirometry may be beneficial to detect pulmonary impairment early in its course. Finally, it can also be said that early diagnosis and appropriate treatment of PTB is important to minimize post-TB fibrosis which may complicate post-TB OAD by worsening overall lung function.
*CXR: Chest X-Ray