There are atleast 15 families of Non-Fermenting Gram-Negative Bacilli (NFGNB). The NFGNB are challenging to the microbiologists and the treating physician because of their intrinsically resistant nature, hardiness and the infection they cause in immunosuppressed patients. NFGNB cause chronic lung infections like cystic fibrosis [1].
The ability to produce β-lactamase enzymes is the major cause of resistance of bacteria to β–lactam antibiotics [2]. There are a number of β-lactamases, which are either chromosome encoded or plasmid encoded. Based on molecular studies, four distinct classes of β-lactamases have been defined namely, classes A and C having serine at their active site, class B (metallo-β-lactamases) having zinc at their active site and class D enzymes or OXA-enzymes which are also serine based but quite distinct from classes A or C [3]. The introduction of carbapenem antibiotics into clinical practice was a breakthrough. Due to their broad spectrum of activity and stability to hydrolysis by most β-lactamases, the carbapenems have been the drugs of choice for treatment of infections caused by penicillin or cephalosporin-resistant gram-negative bacilli [4]. However, this scenario is changing with the emergence of carbapenemase producing strains, especially the NFGNB [5]. Community and hospital acquired infections due to the carbapenemase producing gram-negative bacterial infections are spreading all over the world [6]. Carbapenems are the drugs of last resort for the treatment of multi-drug resistant infections in non-fermenters and other gram negative bacilli [7].
Few Indian studies have documented the presence of carbapenemases in NFGNB. No similar study has been conducted in Irungalur. CLSI 2016 guidelines have been followed in the present study [8]. Studies on KPC CHROMagar for detection of carbapenemases are very few when compared to the other tests like MHT, EDS test and CDT. Hence, the aim of this study was to detect the carbapenemase and MBL producing strains among NFGNB, isolated from clinical specimens in this geographical area by various phenotypic tests and their comparative evaluation.
Materials and Methods
It is a cross sectional study carried out in the Department of Microbiology, Chennai medical college hospital and research center, Irungalur, Trichy between January 2015 and December 2015. Ethical clearance was not sought as the study is based on bacterial isolates only. A total of 5402 clinical samples, which included urine, pus, sputum, blood and body fluids were processed for isolation and identification of NFGNB through standard microbiological assay. All the NFGNB were characterised to the species level using standard laboratory procedures [9].
Patient details like age, gender, ward, clinical diagnosis were obtained from the requisition forms. All the isolated NFGNB were subjected to antibiotic susceptibility testing for gentamycin, amikacin, ciprofloxacin, ceftazidime, cefoxitin, piperacillin, ceftazidime clavulanate, aztreonam and meropenem by disc diffusion assay according to 2016 CLSI guidelines [8].
Meropenem resistant strains were checked for production of carbapenemase and metallo-β-lactamase by the Modified Hodge test (MHT), EDTA Disc Synergy (EDS) test, Combined Disc Test (CDT) and growth on KPC Chromagar.
Modified Hodge Test (MHT): Modified-Hodge test was carried out on Mueller- Hinton agar. From an overnight culture suspension of E.coli (ATCC-25922) (Opacity of the tube was adjusted by comparing with a 1:10 dilution of 0.5 McFarland opacity standard [8]), dipped in cotton swab was inoculated onto the plate as lawn culture. After brief drying, 10 μg meropenem disc was placed at the center of the plate and the test strains were streaked from the edge of the disc to the periphery of the plate and the plates were incubated over-night at 37°C and organisms producing a ‘cloverleaf shaped’ zone of inhibition were identified as carbapenemase producers and interpreted as MHT positive [Table/Fig-1]. Klebsiella pneumoniae (ATCC BAA-1705) and Klebsiella pneumoniae (ATCC BAA-1706) were used as positive and negative controls respectively [8].
Modified Hodge Test (MHT). Clover leaf shaped zone of inhibition-MHT positive (Red arrow); Non-carbapenemase producer (Black arrow).
The bacterial isolate producing a clover shaped zone of inhibition is identified as a carbapenemase producer, and the one which does not produce such indendation is identified as a noncarbapenemase producer.
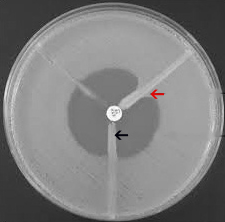
EDTA-Disk Synergy (EDS) Test: For the EDTA-disk synergy test an overnight broth culture of the test strain, (opacity adjusted to 0.5 McFarland opacity standard [8]) was used to inoculate a plate of Mueller-Hinton agar. After drying, a 10 μg meropenem disc and a blank filter paper disk (6 mm in diameter, Whatmann filter paper no. 2) were placed 10mm apart from edge to edge, 10 μl of 0.5 M EDTA (Himedia) solution was then applied to the blank disc, which resulted in approximately 1.5 mg/disc. The plates were incubated overnight at 37°C and an enhanced zone of inhibition was interpreted as EDS positive [10] [Table/Fig-2].
Meropenem-EDTA double disc synergy test. Filter paper disc on right side-Meropenem and disk at centre-EDTA; Enhanced zone of inhibition (Red arrow); No enhanced zone of inhibition (Black arrow). The bacterial isolate producing an enhanced zone of inhibition towards the EDTA disc is identified as a metallo-b-lactamse producer and those that do not produce an enhanced zone were identified as non-metallo-b-lactamse producers.

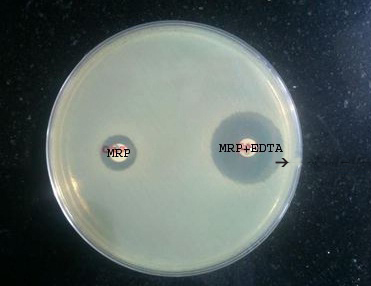
Combined Disc Test (CDT): The test isolates along with standard control strains (opacity adjusted to 0.5 McFarland opacity standard [8] were lawn cultured on MHA plate as recommended by CLSI. After drying, two 10 μg meropenem discs were placed on the lawn culture with 20 mm distance from centre to centre of the discs. A 10 μl of 0.5 M EDTA (Himedia) was added to one of the meropenem discs and incubated overnight. Isolates showing ≥7 mm increase in the inhibition zone size of meropenem-EDTA disc than the meropenem disc alone were considered as MBL producers P [10] [Table/Fig-3].
Meropenem-EDTA combined disc test. Bacterial isolates producing 7 mm or more increase in the diameter of the zone of inhibition with combined meropenem+EDTA disc compared to individual meropenem disc were identified as metallo-β-lactamase producers. (Black arrow).
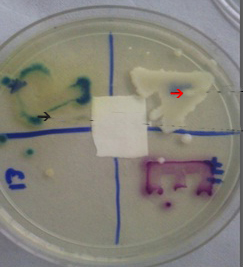
Growth on KPC CHROMagar: The media was prepared with dehydrated powder of CHROMagar KPC (Himedia), which is supplemented with agents that inhibit the growth of gram-positive/gram-negative carbapenem-sensitive bacteria, according to the manufacturer’s instructions. The suspected carbapenemase producing strains were inoculated and incubated for 24 hours at 37°C. Isolates that showed growth on CHROMagar KPC were considered as carbapenemase producers. The carbapenemase producing Pseudomonas and Acinetobacter isolates took on green and cream colours respectively [11]. [Table/Fig-4]. The data were analysed manually by simple statistics and the results were formulated.
Growth on CHROMEagar KPC. Acinetobacter baumanii (Red arrow); Pseudomonas aeruginosa (Black arrow) Bacterial isolates producing growth on KPC chrome agar were identified as carbapenemase producers and those that did not grow were identified as non- carbapenemase producers.
Results
A total of 1302 (24.1%) gram-negative bacilli were isolated from the processed clinical samples, which included 598 (45.9%) NFGNB. The NFGNB were Pseudomonas spp.,(384) Acinetobacter spp.,(176) Stenotrophomonas maltophilia (20) and Burkholderia cepacia (18). Out of the 598 NFGNB isolated from all the 5402 heterogenous clinical samples that were processed, 52 (8.7%) NFGNB showed resistance or intermediate sensitivity to meropenem as tested by disc diffusion assay, which included 30 (57.7%) Pseudomonas aeruginosa, and 22 (42.3%) Acinetobacter baumannii. 21(40.4%) isolates were from pus samples, 18 (34.6%) from urine samples, 10 (19.2%) from sputum samples and 3 (5.8%) from blood samples. 35 isolates (67%) were from males and 17 (33%) from females. Out of 52 bacterial isolates 20 (38.5%) were from patients admitted to medical wards which included male and female medical ward, chest and TB ward, dermatology ward, admitted for conditions like lower respiratory tract infections, post tubercular infections, urinary tract infections and fever, remaining 32 (61.5%) were from patients admitted to surgical wards which included male and female surgical ward, burns ward, surgical intensive care unit and obstetrics and gynaecology ward, mainly for diabetic foot, postoperative infections and infection in burns. 60% of the carbapenem resistant isolates were from males and 40% were from females.
The prevalence of carbapenem resistant NFGNB as detected by our study is 8.7% (52/598). Most of the carbapenem resistant NFGNB were isolated from pus samples (40.4%), and the most commonly affected age group was the above sixty age group (40%). Males (67%) were affected more than females according to our study. Most of the carbapenem resistant NFGNB in our study were isolated from surgical wards (61.5%) and diabetes mellitus was the most common co-morbid condition. All the 52 isolates were resistant to gentamycin, amikacin, ciprofloxacin, ceftazidime, cefoxitin, piperacillin, ceftazidime clavulanate, aztreonam and meropenem when tested by disc diffusion assay according to 2016 CLSI guidelines. All the isolates were susceptible to polymyxin by disc diffusion assay.
Out of the four phenotypic tests that were performed in our study, growth on KPC Chromagar and Modified-Hodge test picked up 94.23% and 55.77% of the carbapenem resistant NFGNB as carbapenemase producers respectively. A total of 88.46% and 84.61% of the carbapenem resistant NFGNB isolates in our study were picked up as metallo-β-lactamase producers by CDT and EDS test respectively as shown in [Table/Fig-5].
Comparative evaluation of carbapenemase production by various phenotypic tests (52 isolates).
| S. No | Phenotypic Tests | Positives (N) | Percentage (%) | Negative Number (N) | Percentage (%) |
|---|
| 1. | Modified-Hodge Test | 29 | 55.77 | 23 | 44.23 |
| 2. | EDTA Disc Synergy Test | 44 | 84.61 | 8 | 15.39 |
| 3. | MRP-EDTA Combined Disc Test | 46 | 88.46 | 6 | 11.54 |
| 4. | Growth on CHROMagar KPC | 49 | 94.23 | 3 | 5.77 |
Discussion
Multi-drug resistant NFGNB are of main concern in hospital settings since they cause multitude of infections [12]. Carbapenemases and metallo-β-lactamases are enzymes that are possessed by several gram negative bacilli, which helps the bacteria in preferential hydrolysis of carbapenems, thereby resulting in drug resistance. Nosocomially acquired carbapenemase producing NFGNB infections pose a real challenge to the clinician, as the organisms resistant to carbapenems are also resistant to other groups of drugs like fluoroquinolones and aminoglycosides being very difficult to treat, resulting in considerable morbidity and mortality among the patients [13].
The prevalence of carbapenem resistant NFGNB as detected by our study is 8.7%, which is concordant with the study by Benachinmardi Kirtilaxmi K et al., [13]. Pseudomonas aeruginosa (57.7%) and Acinetobacter baumannii (42.3%) were the two NFGNB species found to be resistant amongst all the NFGNB isolates that were screened for carbapenem resistance. Most of the isolates were from pus samples (40.4%). This is also concordant with the study by Benachinmardi Kirtilaxmi K et al., [13]. A 67% of the carbapenem resistant isolates were from males especially above 60 years of age. Increased prevalence in males might be due to the non modifiable risk factors like the male gender itself, diabetes mellitus, dyslipidaemia which make them more prone for infections with the resistant bacteria. Elderly patients are more prone for drug resistant infections due to their waning immunity and comorbid conditions [14]. Surgical wards (61.5%) harboured more carbapenem resistant bacteria than medicine and allied wards. All the isolates were resistant to gentamycin, amikacin, ciprofloxacin, ceftazidime, cefoxitin, piperacillin, ceftazidime clavulanate, aztreonam and meropenem as tested by disc diffusion assay according to 2016 CLSI guidelines [8]. The reason for resistance to other groups of drugs is the horizontal gene transfer mechanism which results in transfer of other resistant genes through the same plasmid which carries the gene for carbapenem resistance [15].
Tests which are easy and simple to detect carbapenemase and metallo-β-lactamase are necessary. Currently there is no standardised simple, cost effective yet sensitive method for detection of carbapenemase and MBL producers available [15]. There are a few studies on EDTA disc synergy test, Combined disc test and Modified-Hodge test [15]. According to these studies, carbapenemase production ranged from 7% to 65% [10]. carbapenemase production in NFGNB as detected in our study ranges from 56% to 94% which correlates with the results of Aparna S et al., [10]. Our study has also evaluated the performance of CHROMagar KPC in comparison to the above mentioned tests.
The MHT is a simple test yet has proven to be less sensitive in detecting carbapenemase and MBL production, in our study as only 55.77% of the isolates were positive for MHT. This is concordant with the results of a study by Lee K et al., [16]. Studies have shown that MHT can be made more reliable when carbapenem disc combined with 50 mM ZnSO4 and 0.5 M EDTA is used, which has not been done in our study [16].
A study by Aparna S et al., has showed EDTA disc synergy test to be more sensitive in detecting MBL producers, positivity varying between 14.8 to 72% [10]. 84.61% of the isolates were detected as carbapenemase and MBL producers by EDTA disc synergy test in our study, which correlates well with the study by Tellis R et al., [17]. EDTA disc synergy test is found to be more reliable with high rate of positivity when compared to MHT in our study which is also in concordance with the study by Aparna et al., [10].
However, Combined disc test has picked up four isolates more than EDTA disc synergy test in our study proving to be a better detector of carbapenemase and MBL production than MHT and EDTA disc synergy test. The positivity for carbapenemase production by CDT in our study is 88.46%, which is in concordance with the study by Lee et al., [16]. Previously published data has also proven CDT to be a better detector of carbapenemase and MBL [10].
CHROMagar KPC has shown positivity for carbapenemase and MBL production in 94.23% of the meropenem resistant isolates, which is higher than the other three phenotypic tests performed in our study. The sensitivity and specificity for detecting carbapenem resistant Enterobacteriaceae relative to PCR were 100% and 98.4%, respectively, for CHROMagar KPC according to a study by Samra Z et al., [11]. There are no studies evaluating the effectiveness of CHROMagar KPC for carbapenemase detection in NFGNB. Our study has shown good efficiency of CHROMagar KPC in carbapenemase detection in NFGNB. Thus, growth on CHROMagar KPC has proved to be the most sensitive and reliable test among the four tests and is a better detector of carbapenemase and MBL than MHT, EDS test and CDT.
Limitation
The sample size is low and it is a single centered study. A multi centric study with a large sample size would have been better. The results of four methods for carbapenemase production should have been compared with results of any standard method preferably with PCR or atleast with results of any automated AST system. Lastly the sensitivity of the method ‘growth on CHROMagar KPC’ was shown as 94.23% in the study. But, its specificity is not mentioned or compared. It is possible that this method could have given more false positive results Study that considers all the above points is recommended.
Conclusion
The prevalence of carbapenem resistant NFGNB is only 8.7% in our setting. Even though the prevalence is relatively low, the presence of these resistant bugs itself strongly suggests the need for implementation of strict infection control measures and regular surveillance of the same to prevent further spread of these organisms and also to prevent emergence of further drug resistance among the hospital isolates.
Our study showed that MHT is less reliable for the detection of Carbapemenases and MBLs in NFGNB. EDTA disc synergy test is more sensitive than MHT, but slightly less sensitive than CDT. Hence, CDT is more sensitive and reliable in detection of carbapenemases and MBL than MHT and EDS test. However, growth on CHROMagar KPC is even more sensitive, reliable and detects carbapenemases better. So, CHROMagar KPC has proved to be the best phenotypic test for detection of carbapenemases and MBLs in our study. It is sensitive, cheap, reliable and easy to perform. Therefore, it can be recommended for routine use in clinical laboratories for effective screening of carbapenemase producing bacterial isolates, which can help in accurate and timely detection of drug resistance, so that correct intervention and early directed treatment can be instituted.