Psoriasis is chronic papulosquamous dermatitis with varying incidence between 0.5% to 1.5% of population [1]. Psoriasis is one of the common types of dermatological condition in India with an epidemiological and prevalence characteristics being similar to Western countries [2]. It affects people of all age groups. The mean age of onset is around 25 years although milder form is seen in older persons.
Clinically psoriasis vulgaris which is a chronic plaque type of skin lesion presents as a raised, reddish scaly lesion. PASI score is a scoring tool which measures the psoriatic plaques based on its area of distribution and morphology [3,4]. Histopathologically, psoriasis is characterized by hyperkeratosis, parakeratosis, epidermal hyperplasia, spongiform pustules of Kogoj, suprapapillary thinning of granular layer, inflammatory infiltrate in dermis and capillary proliferation in dermis [5].
The pathogenesis is yet to be elucidated. Various factors seem to play a pathogenic role in psoriasis. They are immune mediated aberrant regulation of T lymphocytes, interaction between rapidly proliferating epidermal cells and an array of cytokines, angiogenesis and vascular remodeling [6]. The inflammatory infiltrate seems to be the prime reason for the whole pathogenesis of psoriasis. There is a significant correlation between the epidermal hyperplasia and inflammatory infiltrate, spongiform pustules of kogoj, and the grade of capillary proliferation [5]. Angiogenesis refers to development of new blood vessels from the pre-existing vascular channels. Immune processes and inflammatory cascades are well known inducers of angiogenesis and vice versa [7]. Studies have suggested that lymphocytes secrete angiogenic factors such as VEGF which induces capillary proliferation and vasodilatation [6,8]. Marked increase in the microvasculature signifies the importance of angiogenesis in the development of psoriasis [9]. There are newer angiogenic factors namely vWF and Nerve Growth Factor (NGF) [9]. These cytokines are said to stimulate angiogenesis in psoriatic skin lesions.
The aim of this study was to analyze and compare the immunohistochemical expression of angiogenic factors - VEGF, vWF and CD 34 in skin biopsies of psoriasis cases with control skin samples; and to correlate the expression of angiogenic factors with psoriasis area and severity clinical index (PASI score).
Materials and Methods
This prospective case control study was conducted over a period of 15 months from April 2013 to July 2014 at Coimbatore Medical College and Hospital, Coimbatore, Tamil Nadu, India. The Ethical Clearance was obtained from the Institutional Ethics Committee.
Thirty-two clinically diagnosed new psoriasis cases between 20 to 65 years of age were selected. Psoriasis patients undergoing any topical or systemic therapy were excluded from the study. Skin biopsies (punch biopsy) were taken under local anaesthesia and aseptic precautions from skin lesions unexposed to sunlight. A 4 mm cylindrical tissue sample was obtained which included epidermis, dermis and subcutaneous fat.
After the procedure, wound was closed by a single suture. Control skin samples included were normal skin present around larger excision biopsies from other departments such as Department of General Surgery at Coimbatore Medical College and Hospital. Patients with psoriasis vulgaris were assessed for severity of lesions by a scoring system known as PASI score.
PASI score is a useful and important tool to calculate the severity of the disease and does not have specific cut off values. It is based on site of distribution (head, trunk, upper limb, lower limb and buttock), morphology (induration, erythema and scaling) and severity of lesions (no symptoms-0, slight-1, moderate-2, marked-3, very marked-4) and percentage of area of distribution (0- 0, 1-9% -1, 10-29% -2, 30-49% - 3, 50-69% - 4, 70-89% - 5, 90-100% - 6) [3,4].
Processing and Staining
The skin biopsy was cut into two portions. The specimens were fixed in 10% formalin, subjected to routine tissue processing and 4-6 μm sections were cut on glass slides for routine Haematoxylin and Eosin staining. The diagnosis of psoriasis was made by analyzing for characteristic histopathologic features as mentioned earlier.
Sections were deparaffinized in xylene and rehydrated in graded alcohol and water. Antigen retrieval was done. Sections were treated with peroxide block followed by application of primary antibody (supplied by biogenex) and super enhancer. Then DAB chromogen was applied with substrate buffer and counterstaining was done with haematoxylin.
Evaluation
The slides were examined under light microscope. The degree of immunohistochemical reactivity for VEGF and vWF were evaluated based on the level of staining of epidermis. They were divided into three groups:
Basal layer only - 1+
Lower half of the epidermis- 2+
Whole epidermis - 3+
VEGF and vWF show cytoplasmic staining. The evaluation of CD 34 staining to assess Microvessel Density (MVD) was done by capillary counting method in three highly vascularized areas (hot spots) selected under 40X magnification.
CD34 shows membranous and cytoplasmic staining. A 40X field was used to count micro vessels in each of the highly vascularized areas. Single endothelial cell or clusters of endothelial cells, either with or without lumen, were considered to be individual vessels [10]. The grading was categorized as follows:
Mild (4-10 capillaries)
Moderate (11-20 capillaries)
Severe (21-28 capillaries)
Statistical Analysis
The data were reported as the mean±SD or the median, depending on their distribution. The differences in quantitative variables between groups were assessed by means of the unpaired t-test. Comparison between groups were made by the non parameteric Mann-Whitney test. A chi-square test was used to assess differences in categoric variables between groups. Pearson’s coefficient of correlation was used to assess the relationship between the variables. A p-value of <0.05 was considered significant for all statistical tests.
Results
Among the cases, maximum number of patients belonged to 41 to 50 years of age (13 cases, 41%). In the present study, among the cases male to female ratio was 1.2:1 (18 males and 14 females). Most of the cases belonged to the PASI score more than 17 (14 cases). The mean PASI score in the present study was 16.2±1.9.
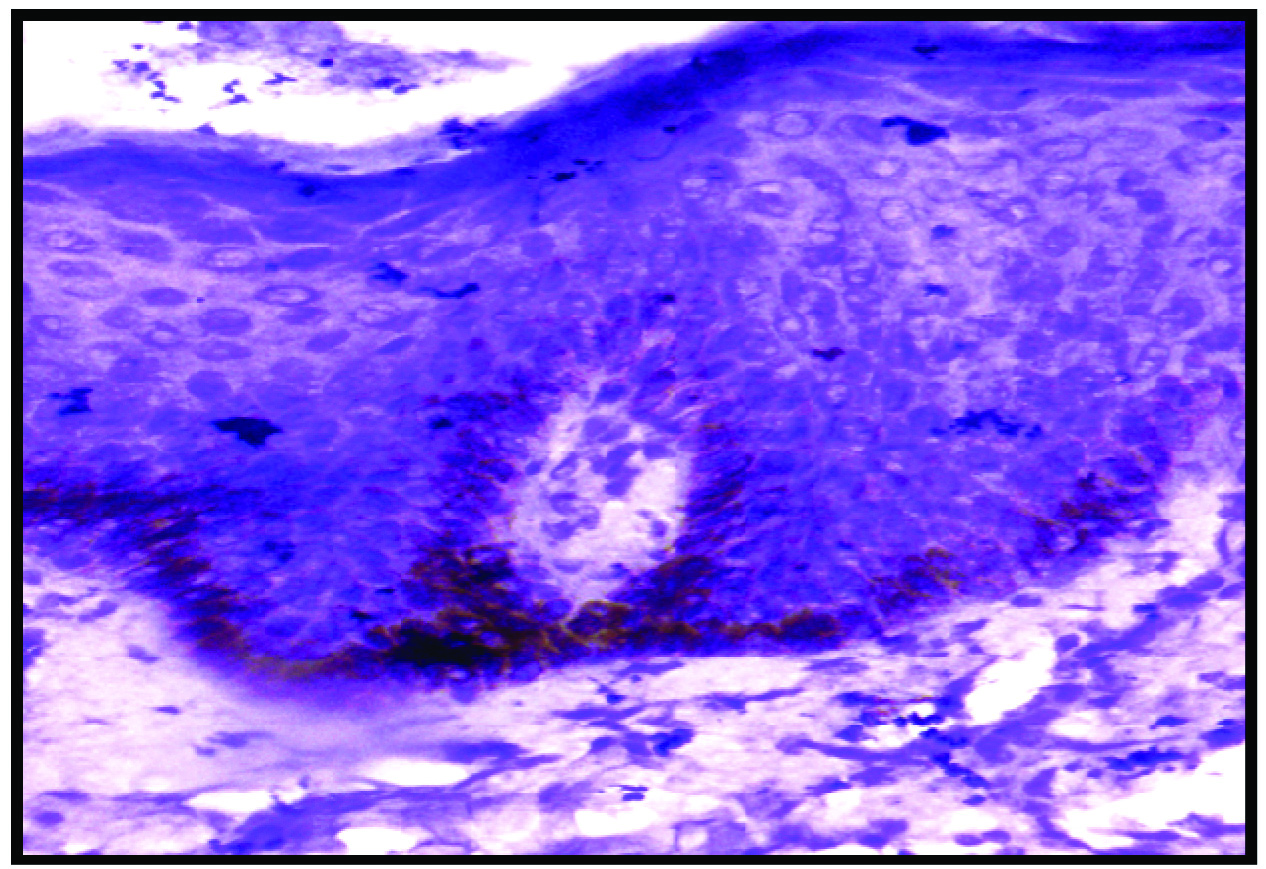
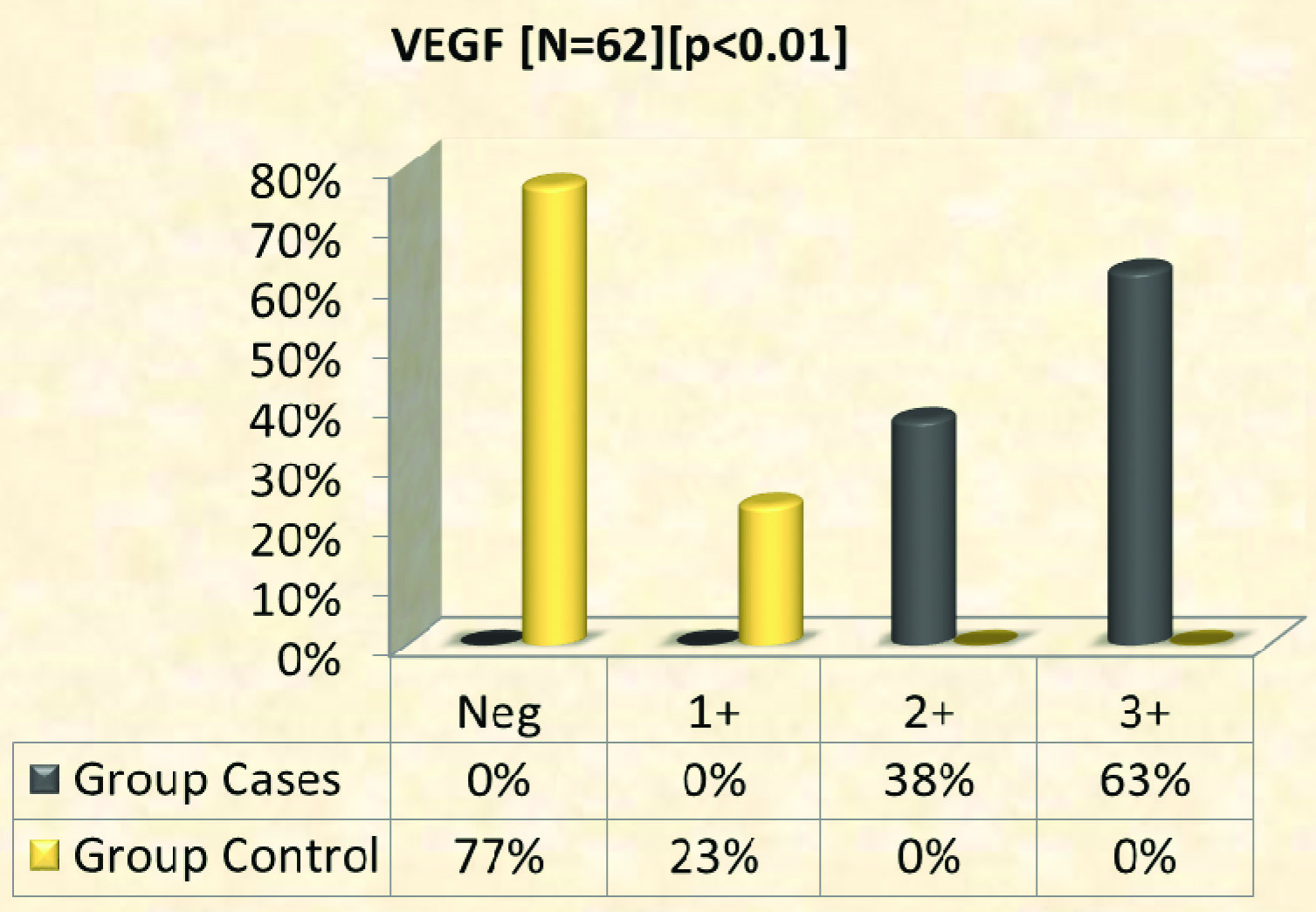
Expression of VEGF: VEGF expression was seen in all cases and weak staining in controls [Table/Fig-1,2,3,4 and 5]. A total of 63% of cases showed full thickness epidermal staining and 23% of controls showed weak positivity of epidermis [Table/Fig-6]. Mean expression of VEGF among cases was 2.6±0.5 SD and among controls 0.2±0.4 SD.
Expression of VEGF; moderate degree of epidermal positivity (2+) in cases (IHC, 10X).
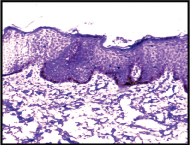
Expression of VEGF; weak epidermal staining (1+) in controls (IHC, 10X).
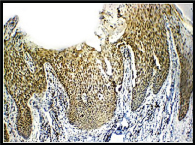
Expression of VEGF; diffuse epidermal positivity (3+) in cases (IHC, 10X).
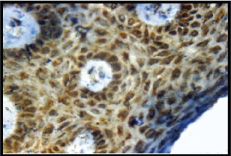
Expression of VEGF; cytoplasmic and membranous positivity (3+) in epidermis in cases (IHC, 40X).
Expression of VEGF – weak positivity in epidermis (1+) in controls (IHC, 40X).
Immunohistochemical expression of VEGF in cases (n = 32) and controls (n = 30).
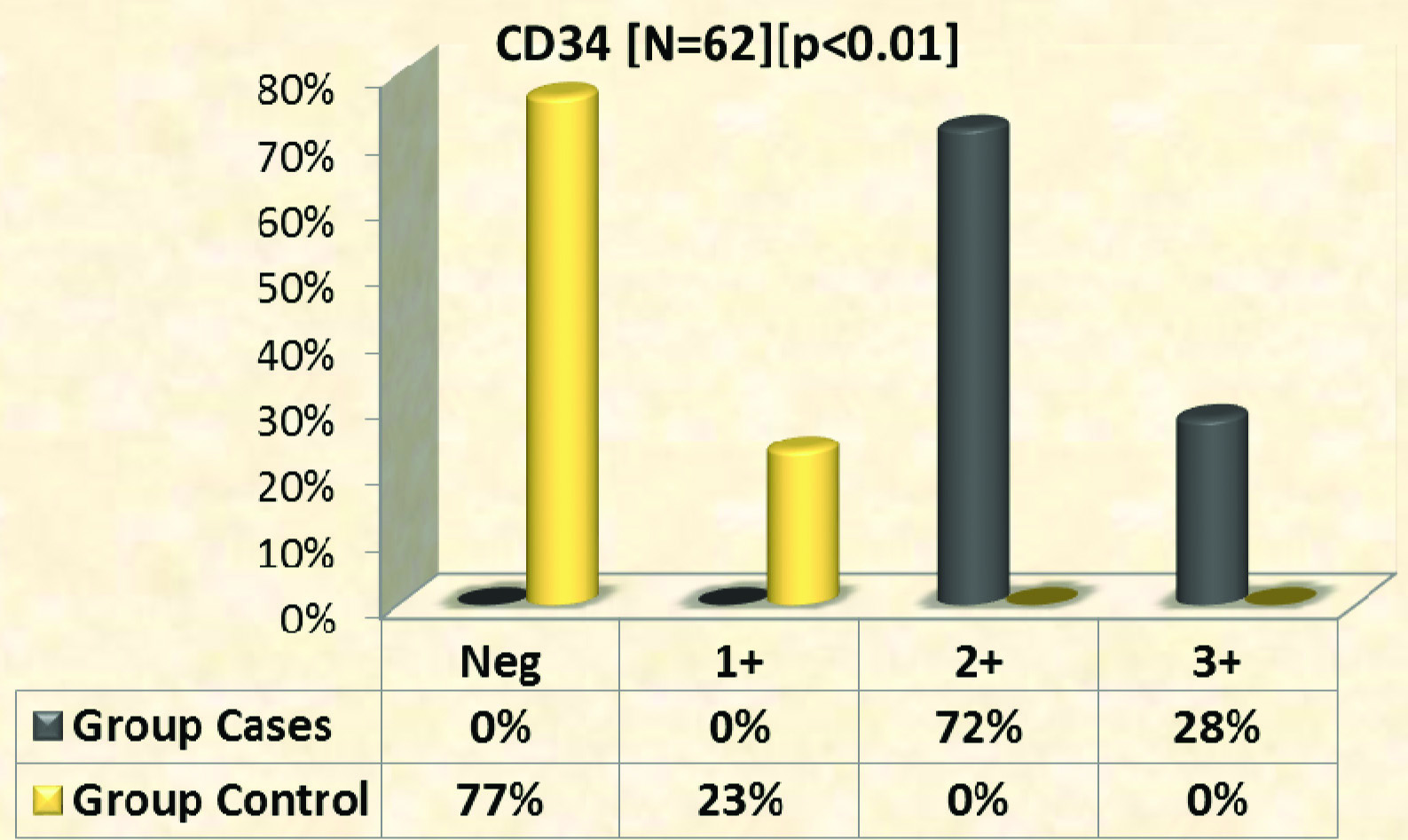
Expression of CD 34: CD 34 expression with various intensities was seen in all samples from psoriatic skin lesions [Table/Fig-7,8]. Among controls [Table/Fig-9] 23% showed weak positivity and among cases 72% of skin samples showed moderate degree of staining and 28% with higher intensity of staining [Table/Fig-10]. Mean expression of CD 34 among cases was 2.3±0.5 SD and among controls 0.2±0.4 SD.
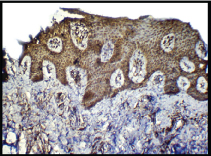
Expression of CD 34; higher degree of positivity (3+) in cases (IHC, 10X).
Expression of CD34; higher degree of positivity (3+) in cases (IHC, 40X).
Expression of CD34; weak positivity (1+) in controls (IHC, 40X).
Expression of CD 34 in cases (n = 32) and controls (n = 30).
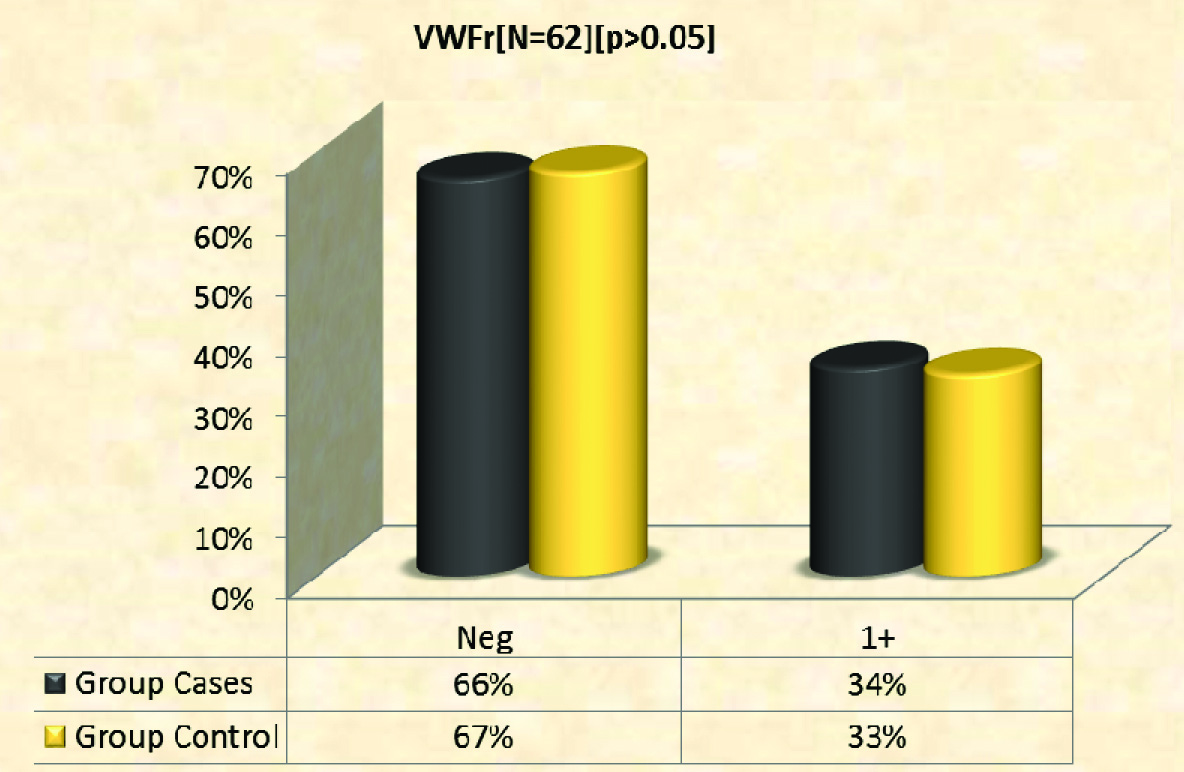
Expression of vWF: von Willebrand factor showed weak epidermal expression in both cases and control group [Table/Fig-11,12]. Percentage of negative staining was 66% and 67% in cases and controls respectively [Table/Fig-13]. Mean expression of vWF among cases was 0.34±0.48 SD and among controls 0.33±0.48 SD.
Expression of von Willebrand factor; weak epidermal positivity (1+) in cases (IHC, 40X).
Expression of von Willebrand factor; weak epidermal positivity (1+) in controls (IHC, 40X).
Immunohistochemical expression of vWF in cases (n = 32) and controls (n = 30).
Coefficient of correlation: There was statistically significant correlation between PASI score and expression of VEGF (n=32; r=0.944; p<0.01) and CD 34 (n= 32; r=0.942; p<0.01). There was no statistically significant correlation between expression vWF with PASI score (n= 32; r=0.024; p>0.05) [Table/Fig-14].
Correlation between PASI score and angiogenic factors.
| | PASI Score | CD 34 | VEGF | vWFr |
|---|
| PASI Score | Pearson Correlation | 1 | 0.942 | 0.944 | 0.024 |
| Sig. (2-tailed) | | 0.000 | 0.000 | 0.855 |
| N | 62 | 62 | 62 | 62 |
| CD 34 | Pearson Correlation | 0.942 | 1 | 0.879 | 0.058 |
| Sig. (2-tailed) | 0.000 | | 0.000 | 0.653 |
| N | 62 | 62 | 62 | 62 |
| VEGF | Pearson Correlation | 0.944 | 0.879 | 1 | -0.022 |
| Sig. (2-tailed) | 0.000 | 0.000 | | 0.866 |
| N | 62 | 62 | 62 | 62 |
| vWFr. | Pearson Correlation | 0.024 | 0.058 | -0.022 | 1 |
| Sig. (2-tailed) | 0.855 | 0.653 | 0.866 | |
| N | 62 | 62 | 62 | 62 |
Discussion
Psoriasis has a complex pathogenesis [11] characterized by altered keratinocyte proliferation and differentiation, immune mediated inflammation, dysregulated angiogenesis and vascular remodeling [4]. Various factors which play a central and pivotal role in the patho-mechanism of psoriasis are Th1 type of cell, Th17 cell, antigen presenting cell, Langerhans type of cells, natural killer cell, keratinocytes and macrophages and various cytokines of Th1 type [11]. New blood vessel formation is seen in early stages of psoriatic lesions and neo-vascularization disappears with clearance of skin disease [12]. Hence, angiogenesis is not only a cofactor but induces development as well. The expression of proangiogenic cytokines like tumour necrosis factor, VEGF, hypoxia inducible factor, Interleukin 8 and angiopoietins, are increased in lesional skin of psoriasis [13,14]. Keratinocyte is said to be one of the main source of proangiogenic cytokines. Evidence for keratinocytes producing proangiogenic signals originated from an observation in a study that compared the angiogenic property of conditioned media from epidermal keratinocytes obtained from either psoriasis skin lesion or non lesional skin of psoriasis patients [15]. In psoriasis vulgaris, ultrastructurally the activated endothelial cells show enlarged Golgi bodies and Weibel Palade bodies [16]. These cells migrate, sprout, and lay a basement membrane with pericytes which forms a structural support for new vessels [17]. They also widen the inter-cellular spaces and cause dilatation of capillaries. The increased proliferation of endothelial cells in psoriatic plaques [18,19] has been proved by autoradiography and immunohistochemical studies [20]. Psoriasis skin lesions begin with neoangiogenesis in superficial dermis. Dermal papillary capillaries show prominent dilatation, increased tortuosity, permeability, and prominent elongation [18,21,22]. These morphological changes occur before epidermal hyperplasia becomes evident [21,23]. The microvascular changes in the early stages of psoriatic lesions correlates with increased cutaneous vascular flow and similar changes are also seen in the neighbouring perilesional areas [24].
Ultrastructurally in psoriasis, the capillaries are of venous type (single or multilayered basement membrane with bridging of fenestrations in the endothelium) whereas, in normal skin the capillaries are said to be arterial type [25]. Venous capillary type converts to arterial capillary type after therapy. Normalization of the superficial dermal vasculature and capillary loops is followed by normalization of epidermis [18].
Vascular Endothelial Growth Factor (VEGF)
VEGF and their high affinity tyrosine kinase receptor vascular endothelial growth factor receptor -1 and -2 (VEGFR-1, VEGFR-2) are primarily involved in vessel embryogenesis and adult new vessel formation. VEGFR-1 and VEGFR-2 are expressed by endothelial cell. Vascular endothelial growth factor binding to VEGFR 1 or 2 receptors results in activation of receptors and intracellular signal transduction [26–28]. VEGF induced cell proliferation, survival, migration, and increased vascular permeability are essentially transduced by VEGFR-2 [29]. VEGF induced angiogenesis contributes to the patho-mechanism of psoriatic skin lesions. In situ hybridization and immunohistochemical studies reveal that there is a strong upregulation of VEGF mRNA and expression of its proteins in the cells of epidermis along with increased expression of VEGFR 1 and VEGFR 2 on endothelial cells which are present in the dermis of psoriasis skin lesions [30]. Serum from patients with psoriatic skin lesions showed increased VEGF levels and the levels correlates with disease severity [31–33]. The increased expression of VEGF shown by single nucleotide polymorphisms of the vascular endothelial growth factor in psoriasis suggests that VEGF plays a prime role in the aetiopathogenesis of psoriatic skin lesions [34,35].
Selective overexpression of VEGF in basal keratinocytes lead to a long standing inflammatory dermatological disease with increase in number of dilated tortuous capillaries expressing enhanced levels of VEGFR 1 and VEGFR 2, higher number of mast cells in the superficial portion of the dermis and enhanced leukocyte rolling and adhesion [36,37]. Apart from its central role which causes aberrant angiogenesis in upper portion of the dermis, vascular endothelial growth factor also contributes to keratinocyte proliferation and homeostasis of epidermal barrier [38,39]. Psoriasis can also be aggravated by external injury which is known as Koebner phenomenon and the damage to the epidermal barrier homeostasis stimulates vascular endothelial growth factor expression [40].
In the present study the average PASI score for cases was 16.2±1.9. In a similar study done by Liew SC et al., the average PASI score was 7.247±4.780 [9].
In our study, VEGF expression was observed in all cases with various intensities. VEGF expression was weak in most of the controls. VEGF expression was significantly higher in cases compared to controls with a statistical significant difference (p=<0.05). Similar results were observed in the following studies. According to a study conducted by Liew SC et al., the intensity of VEGF expression was higher in cases compared to controls (p=0.016) [9]. Simonetti O et al., observed that there was diffuse VEGF (13.15±6.6) immunohistochemical expression in epidermis of psoriatic skin lesions compared to epidermis of normal skin [6]. In another study done by Rashed HE et al., there was strong VEGF expression in epidermis (mean 46.1±19.66) and a moderate expression in vessels and inflammatory infiltrates (mean 19±5.4 and 8±2.16) [37]. VEGF expression was significantly higher in skin of psoriasis cases compared to normal healthy controls [17]. In a similar study conducted by Kim YG et al., the expression of VEGF was significantly enhanced in skin of psoriasis cases when compared to controls [40]. The above studies correlate with our study and support that VEGF promotes endothelial cell survival, new blood vessel formation and thus plays a significant role in pathogenic basis of psoriasis. Bhushan M et al., reported that VEGF was mainly produced by epidermal keratinocytes compared to fibroblast [32]. In the present study there was significant correlation between the expression of VEGF with PASI score (r=0.944, p=<0.01). In a study conducted by Liew SC et al., there was no significant correlation between PASI score and VEGF (p=0.232) [9]. In the present study, expression of CD 34 was observed in all samples of psoriasis with variable intensities, whereas CD 34 expression in controls was weak. There was significantly higher expression of CD 34 in cases compared to controls which is statistically significant (p=<0.01). In a study done by Amin MM et al., they observed that CD 34 expression was higher in cases when compared to controls and non lesional skin [10]. In another study by Gupta S et al., showed that the microvessel density was higher in skin of psoriasis (71.28±40.05) [41]. A study conducted by Barton SP et al., showed that there is higher endothelial cell volume and volume of lumen in the skin of psoriatic lesions compared to the non lesional skin of psoriasis and healthy skin of controls [42]. In a study done by Simonetti O et al., CD 34 was expressed significantly higher in skin of psoriatic lesions compared to controls (19.15±12.61 vs 3.0±0.23; p=0.04) [6]. The above studies support our observation and indicate that in psoriasis there is vascular proliferation, tortuosity and elongation of vessels in response to inflammation reflected by increased microvessel density. In the present study there was significant correlation between the expression of CD 34 with PASI score (r=0.942; p=<0.01). In a similar study by Guijiao BI et al., indicates that CD 34 expression was increased in vascular endothelial cells in dermis of psoriatic skin lesions and might be related to severity of psoriasis [43,44]. CD 34 might also be involved in adhesion and migration of inflammatory cells. In the present study, expression of vWFr both in lesional skin and control skin was weak. There was no statistically significant difference (r= 0.024; p=>0.05). In a similar study conducted by Liew SC et al., there was no expression of vWFr in psoriatic cases [9]. In the present study there was no statistically significant correlation between PASI Score and VWFr (p=>0.05). In a similar study conducted by Liew SC et al., there was no significant correlation between PASI score and vWFr (p=0.169) [9].
The above studies indicate that angiogenesis plays a key role in pathogenesis of psoriasis. Other evidences which support that angiogenesis are seen in psoriasis are the following:
Enhanced blood flow in psoriasis skin biopsy using laser Doppler fluxmetry.
Increased microvasculature in the psoriatic skin lesion seen in autoradiograph.
The ultrastructural studies which show tortuous and elongated capillary loops in lesional psoriatic skin [45].
Limitation
In this study the expression of angiogenic factors was not studied on skin biopsies of treated psoriasis patients and non lesional skin of psoriasis patients. However future research with larger samples and newer angiogenic markers could be done to analyze in detail the angiogenic pathways in psoriasis for the development of targeted anti-angiogenic therapy.
Conclusion
Psoriasis is a chronic inflammatory dermatological and disabling disease. Both the keratinocytes and the T lymphocytes secrete angiogenic factors which are responsible for neo-vascularization in psoriasis. Angiogenesis and inflammatory infiltration might be interdependent in psoriasis. Hence, antiangiogenic therapy might be a useful therapeutic approach in psoriasis.