Granulocytic Sarcomas (GS) also called as Myeloid Sarcomas (MS) or chloromas are the representatives of extramedullary infiltrates of immature myeloid cells including myeloblasts, promyelocytes and myelocytes. Primary cardiac malignancies per se are rare and infiltration of cardiac muscles by secondary malignant cells is also an uncommon finding. Out of these cardiac tumors, contribution of Cardiac Myeloid Sarcoma (CMS) is even more smaller thereby limiting our knowledge about this rare entity. Because of its very lower incidence, an exact guideline for diagnosis and management is still missing and usually haematologists around the world are treating CMS based on their clinical acumen. Aim of this review is to briefly discuss the presenting clinical feature, differential diagnosis, diagnostic workup and management based on published articles related to CMS till date.
Introduction
GS is most commonly associated with Acute Myeloid Leukaemias (AML), but can be also seen in association with Myeloproliferative Neoplasms (MPN) or Myelodysplastic Syndromes (MDS) [1]. Very rarely, it has been reported in chronic myelogenous leukaemia presenting in blast crisis. The usual reported location of chloroma is skin, bone, periosteum, spleen and soft tissues. However, acute leukaemias especially AML can have atypical presentations and we have reported rare areas of involvement such as orbit, pleura, mediastinum and spinal cord in recent past [2–7]. Presence of CMS either as locally invasive or isolated cardiac intracavitatory mass is highly uncommon. The occurrence has been reported anytime during the entire disease course i.e., before the diagnosis, during treatment, post chemotherapy relapse or post stem cell transplant [8]. In most cases, symptoms are similar to that of classical commoner cardiac illnesses [9]. Both the diagnosis as well as management is challenging and must be tailored based on clinician’s experience and patient’s performance status. MS in other common locations is well known findings in AML and treatment protocols have also been laid down. In view of its rarity of occurrence, diagnostic limitations and restricted of use of conventional anti-leukaemia agents (due to the fear of cardio toxicity), the same guidelines cannot be applied to CMS. This review aims to highlight the challenges which haemato-oncologists do face while dealing with such cases.
Materials and Methods
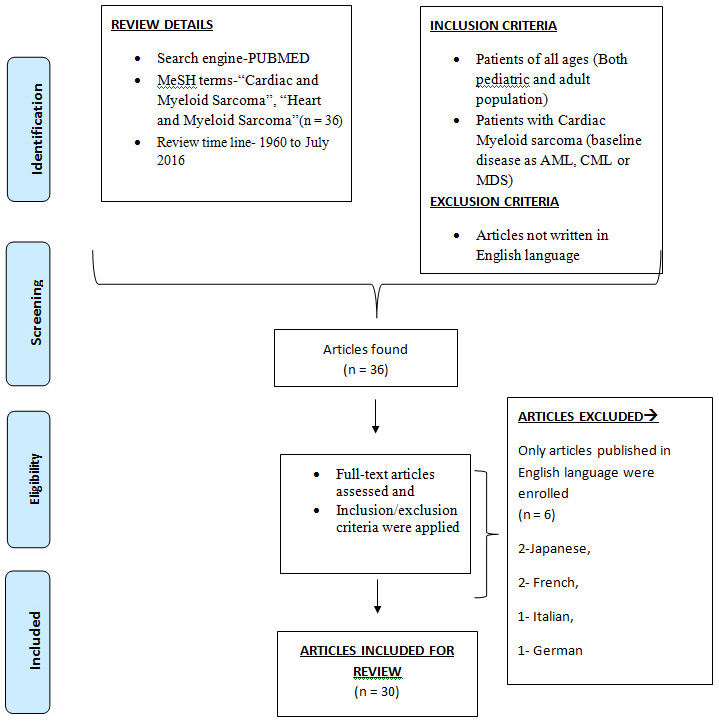
We did a MEDLINE/PubMed search with MeSH terms–(“Heart” and "Myeloid Sarcoma") and "(Cardiac and Myeloid Sarcoma)". In total, we found 36 articles reported (From 1960 to July 2016). For a comprehensive review, we have included all the cases including both paediatric and adult population. Exclusion criteria was removal of reports from review which were written in languages other than English. Hence, six cases (written in Japanese, French, Italian, and German language) were excluded. Hence, finally a total number of 30 articles were included in this study [Table/Fig-1]. Detailed summary of all the articles included in the study is mentioned in [Table/Fig-2] [1,8–36].
Review of published cases of cardiac chloromas in literature from 1960 till July 2016, their baseline disease characters, molecular characteristics, diagnostic modality used, areas of cardiac tissue involved with outcome [1,8–36].
| Author and year of publication | Age /sex | Disease/FAB Subtype | Molecular Marker/FISH/Cytogenetics | Cardiac chloroma at diagnosis or relapse | BM involvement | Diagnostic modality used | Pathological areas involved | Outcome |
|---|
| Wang Y et al., [10] | 66/M | AML | NA | At diagnosis | + | PET scan 2D Echo | Ascending aorta, Pulmonary artery, pericardial effusion, B/L atrial enlargement | NA |
| Niu N et al., [11] | 66/M | AML | NA | At diagnosis | + | PET scan 2D Echo | Both ventricles and atria, pericardium | NA |
| Yang WC et al., [12] | 19/M | AML(Post allo-HSCT) in CR | Normal Karotype, NPM-ve) | At relapse | NA | CT scan PET scan | Soft tissue mass along the interatrial groove with an encasement of the pulmonary vein causing SVC syndrome | IMRT (24 Gy in 12 fractions) and Azycytidine following which achieved complete remission. Died soon due to sepsis. |
| Dorfel D et al., [13] | 69/M | AML | FLT3 + ve NPM1+ve | At relapse | - | 2D Echo CEMRI VPCT | Epicardial mass infiltrating right ventricular wall. Another mass at coronary sulcus | Died due to sigmoid diverticulitis and MODS |
| Schaffer LR et al., [14] | 7/M | AML-M 5 | Not mentioned | At diagnosis | + | CXR 2D Echo | Subtotal replacement of the myocardium, sinoatrial and atrioventricular (AV) nodes | Died |
| Kim JG et al., [15] | 41/M | CML (Post MUD-HSCT) in CR | BCR-ABL 1 status not mentioned | At relapse | + | 2D Echo CEMRI | Irregular shaped mass in the right atrium | Size of cholorma increased after 17 months. Dose of Dasatinib was increased to 140 mg/day |
| Mawad R et al., [16] | 42/M | Not applicable | t(8;21) 46XY | At diagnosis | - | 2D Echo PET scan | Bilateral atrial walls, intra –atrial septum | Complete remission Non FDG avid residual mass |
| Cash T et al., [17] | 24/F | AML /M5 | 46,del(x) Trisomy 21 | At relapse | - | 2D Echo CECT chest CEMRI | Mass adhering to right ventricle with RVOT obstruction pericardial effusion | Died within four months of achieving CR1 due to relapse of choloroma at the same time |
| DI Valentino M et al., [18] | 38/F | AML | Not available | At relapse | - | 2D Echo CEMRI | Infiltration of right free wall with RVOT obstruction | Complete remission |
| Makis W et al., [19] | 42/M | AML | Not available | At diagnosis | NA | CT scan PET | Mediastinal mass with invasion of the parietal pericardium | Pericardial therapeutic drainage and HiDAC therapy- Did not achieved remission. Post two months PET showed disease progression. |
| Tirado CA et al., [20] | 30/M | APML | +7p22, t(15;17) | At relapse | NA | | Multiple sites- (left scapula, thoracic vertebra, right atrium, and supraclavicular mass | NA |
| Atallah A et al., [21] | 56/M | AML | Not available | At diagnosis | + | 2D Echo CEMRI | Focal, patchy, epicardial infiltrative process affecting mid-inferior wall | Died due to chemoresistant leukaemia |
| Tsai J et al., [22] | 20/M | AML | Not available | At relapse | - | 2D Echo MDCT | Well marginated, lobulated mass involving the intra-axial septum and both atria. Another mass located near the posterior aspect of tricuspid valve | Complete remission |
| Matkowskyj KA et al., [23] | 59/M | T-MDS – RAEB type (treated 3 years back for Anaplastic oligodendroglioma) | Abnormal mosaic male karyotype (46,XYand 45,XY,-7) | At diagnosis | - | 2D Echo | Left ventricle, septum, and right ventricle | Died before treatment could be started |
| Mignano JE [24] | 20/M | AML, FAB M-2 | Not mentioned | Relapse | - | CT | Right and left atria, intra-atrial septum, and apparent extension into the right pulmonary veins | Resolution |
| Tsai MH [25] | 1.4 mon/F | AML | 51,XX,add(1)(p32),del(4)(q31),+del(6)(q23),add(7) (q11.2),+8,+8,der(11)t(7;11)(q11.2;q23),+13, add(16)(q24), +19. | At diagnosis | + | CXR, ECG | Pericardium invasion | Died secondary to sepsis |
| Rigamonti F et al., [27] | 52/M | AML | 47,X,add(Y)(q12) | At diagnosis | - | Cardiac Ultrasound | Infiltration of the infero-lateral cardiac wall, right auricle and aortic arch | 3+7 Regimen/ Patient died despite achieving remission due to fungal sepsis |
| Kozelj M [26] | 52/M | AML | t(8;21), | At diagnosis | + | 2D Echo, TTE, MRI | Right atrium, right pulmonary hilum | Remission |
| Antic D et al., [28] | 37/M | AML | NA | At diagnosis | - | CT Chest | Large mass in right atrium extending into SVC causing SVC syndrome | Surgical excision of the mass followed by 3+7 regimen |
| Diab M et al., [29] | 27/M | AML | NA | At diagnosis | - | CT Chest | Leukemia cutis, Pericardial effusion, mediastinal mass | Pericardiocentesis, Cytrabine, Daunorubicin and etoposide based regimen. Refractory to chemotherapy due to which patient soon. |
| Nasilowska AB et al., [30] | 34/M | APML in CR (Post allo-HSCT) | t(15;17) | At Relapse | - | CT Chest | Multiple sites of EM relapse- pleura, heart and pericardium | During salvage chemotherapy, patient died of cardiogenic shock |
| Mateen FJ et al., [1] | 64/W | MDS, RAEB | Not mentioned | At diagnosis | + | CXR, 2D echo | Myocardium infiltration | Died |
| Antón E et al., [31] | 45/F | Not applicable | +4, -9, -17, add(17)(p13), -18, +21, +22 | At diagnosis | - | CXR, ECG | Myocardial infiltration | Died due to multi organ failure |
| Kara IO et al., [8] | 28/M | AML, FAB-M2 in CR 2 (Post allo- HSCT) | Not mentioned | At relapse | + | 2D Echo | Nasopharynx nodular masses, right atrium, right ventricle | Etoposide, mitoxantrone, ara-C (EMA) chemotherapy regimen- achieved CR |
| Marcos AP et al., [32] | 39/M | AML, FAB-M1 | Not mentioned | At relapse | - | TEE | Right atrium | Complete remission |
| Markaryus AN et al., [33] | 34/M | AML, FAB-M1 | Not mentioned | At relapse | + | TTE, 2D echo, TEE, MRI | All chambers | Died |
| Erdol C et al., [34] | 48/M | AML, FAB-M4 | Not mentioned | At diagnosis | + | TTE, TEE, Echocardiography | Right atrium | Complete regression post chemotherapy |
| Tillawi IS et al., [35] | 72/M | Angiogenic myeloid metaplasia | | | NA | | Encasement of the heart and great vessels | |
| Jankovic M et al., [9] | 12/F | APML | Not done | At relapse | - | CXR, Echocardiography | Right-sided intracardiac mass, pericardial effusion | Died due to progressive heart failure |
| Foucar K et al., [36] | 22/M | Not applicable | Not done | At diagnosis | - | CXR, Echocardiography | Encasement of the SVC, PA, and aorta | Died due to ventricular arrhythmia |
Discussion
Underlying Basic Disease
We found that most of the cases reported had AML as the basic disease followed by Acute Promyelocytic Leukaemia (APML), MDS, CML and angiogenic myeloid metaplasia. The average age of patients was 40 years (ranging from 1.4 months to 72 years). There were in total 26 males and five females (including three children under the age 12 years). There were also four cases of post allogeneic transplantation that developed CMS during post-transplant period follow up [8].
Symptomatology
Review showed that CMS can present with a wide array of symptoms. An excellent review by Lam KY et al., of over 12000 consecutive autopsies done over the span of 20 year period showed the incidence of cardiac involvement by leukaemia of just 4% [37]. A majority of these remain undiagnosed in living individuals and discovered subsequently only on autopsy. In the symptomatic individuals, pericarditis is the frequent presentation with or without leukaemic cells in pericardial fluid [25]. Other clinical findings may include tachycardia, dyspnoea on exertion, palpitations, chest pain, arrhythmias, heart block, etc. Review showed that symptomatology of CMS in general is no more different from that of classical cardiac diseases like CAD, cardiomyopathies etc. Recognition of subtle clinical signs without frank heart failure like elevated jugular venous pressure with tall “a” wave, pedal oedema and tender hepatomegaly is crucial to warrant further imaging and invasive tests.
Diagnosis and Investigative Modalities
Diagnosis of GS requires high index of suspicion and definitive diagnosis can be very laborious and unyielding. Gold standard diagnosis is histological examination of the endomyocardial biopsy specimen [26]. Yield of biopsy samples can be increased by combining with newer diagnostic tests like MRI (SSFP/FLASH MRI) and PET scan. Superiority of PET scan is due to high efficacy in finding unifocal v/s multifocal involvement, in assessing the most 18 Fludeoxyglucose (FDG) avid site for biopsy and the treatment response after chemotherapy. Various diagnostic modalities and their utilities are summarized in [Table/Fig-3] [32,33].
Diagnostic modalities and their efficacy in diagnosing.
| ECG, Chest X Ray | Non-specific test, cheap, beneficial to give quick idea about arrhythmia, effusion and cardiac borders and mediastinal involvement. |
| 2D - Echocardiography and TEE | Fast and dynamic, TEE can be used to take biopsy. |
| CT Scan | Quick, easily available. To analyze intra-cardiac invasion and involvement of pulmonary vasculature. Volume perfusion scan (VPCT) to assess viable myocardium. |
| MRI | Steady state free procession imaging (SSFP MRI) and fast low angle short magnetic resonance imaging (FLASH MRI) can acquire dynamic images synchronised with ECG. |
| PET Scan [40] | Best to assess the site for biopsy and the treatment response. |
A detail histopathological examination including light microscopy, immunohistochemistry and Fluorescence in Situ Hybridization (FISH) should be done on biopsy samples. The common histopathological features are highly cellular, diffuse proliferation of medium-size, polygonal to rounded, polymorph, undifferentiated malignant cells, with a high nuclear-cytoplasmic ratio, high mitotic index, scattered to diffuse areas of necrosis, and irregular production of finely reticular to coarse fibrinous tissue [34]. The cytoplasm is usually eosinophilic and granular. Positivity for CD 34, 45, 17 and HLA-DR indicates tumor mass to be of myeloid origin. Combined team effort of radiologists, cardiologists and haematologists to diagnose and manage these challenging cases is of utmost importance.
Management Challenges
There is no consensus on the type of regimen to be used in CMS cases and haematologists worldwide have used various regimens depending on patient’s tolerability and cardiac compromise. Anthracyclines are considered as backbone in the treatment therapy of AML, however the same might not be suitable in all cases due to risk of potential cardiotoxicity. Etoposide, Mitoxantrone, Cytarabine/Ara-C (EMA) based regimens have been shown to be partially efficacious in the treatment of CMS in a few case reports [35,38] Milder forms of therapy e.g., hypomethylating agents like azacytidine have also been tried which makes chemotherapy possible even in patients of older age, poor performance status and with compromised cardiac function [12,39]. A combination chemotherapy and minimal residual disease targeted therapy is needed to control the disease locally and to prevent bone marrow relapse. Newer radiotherapy protocols based on IGRT and IMRT have also broadened the treatment options where in only pathological part of heart are radiated thereby preserving the cardiac function. A total of 24 Gy in divided doses (12 fractions) was used by Yang WC et al., with successful remission achievement [12].
Conclusion
Despite various chemotherapeutic and radiation based treatment protocols, CMS carries a dismal prognosis. High mortality and morbidity is associated with this rare disease. Major limitation is that anthracycline based regimens cannot be used due to their own cardiotoxicity. Further research is warranted to identify newer molecular markers for diagnosis, standardize diagnostic tests and formulate efficacious chemotherapy and radiation protocols.
[1]. Mateen FJ, Harding SR, Saxena A, Extensive myocardial infiltration by hemopoietic precursors in a patient with myelodysplastic syndromeBMC Blood Disord 2006 6:4 [Google Scholar]
[2]. Sahu KK, Yanamandra U, Malhotra P, Orbital myeloid sarcoma: Rare presentation of AMLOrbit 2016 35(3):157-58. [Google Scholar]
[3]. Sahu KK, Malhotra P, Re: “Granulocytic sarcoma of the orbit presenting as a fulminant orbitopathy in an adult with acute myeloid leukaemia”Ophthal Plast Reconstr Surg 2015 31(5):421 [Google Scholar]
[4]. Sahu KK, Jain A, Yanamandra U, Varma SC, Malhotra P, Myeloid sarcoma of vulva: a short updateIndian J Haematol blood Transfus an Off J Indian Soc Haematol Blood Transfus 2016 32(Suppl 1):69-71. [Google Scholar]
[5]. Jain A, Sahu KK, Sharma S, Rajwanshi A, Suri V, Malhotra P, Shoulder myeloid sarcoma: an initial presentation of cml blast crisisIndian J Haematol blood Transfus an Off J Indian Soc Haematol Blood Transfus 2016 32(Suppl 1):361-63. [Google Scholar]
[6]. Sahu KK, Sanamandra P, Jeyaraman P, Kumar G, Prakash G, Kumar N, Unusual cause of cord compression-A pressing issue for neurosurgeonsWorld Neurosurg 2016 92:565-67. [Google Scholar]
[7]. Sahu KK, Tyagi R, Law AD, Khadwal A, Prakash G, Rajwanshi A, Myeloid sarcoma: an unusual case of mediastinal mass and malignant pleural effusion with review of literatureIndian J Haematol blood Transfus an Off J Indian Soc Haematol Blood Transfus 2015 31(4):466-71. [Google Scholar]
[8]. Kara IO, Sahin B, Paydas S, Kara B, Granulocytic sarcoma of the heart: extramedullary relapse of acute myeloblastic leukaemia after allogeneic stem cell transplantation successfully treated by chemotherapy aloneLeuk Lymphoma 2005 46(7):1081-84. [Google Scholar]
[9]. Jankovic M, Bonacina E, Masera G, Uderzo C, Galli MA, Ottaviani V, Cardiac relapses in myeloid leukaemia: case report and review of the literaturePediatr Haematol Oncol 1987 4(3):237-45. [Google Scholar]
[10]. Wang Y, Wu W, Zhong D, Han X, Fang L, Multimodality imaging and histopathology of cardiac myeloid sarcomaEur Heart J Cardiovasc Imaging 2016 17(11):1316 [Google Scholar]
[11]. Niu N, Cui R, Li F, FDG PET/CT findings of intracardiac myeloid sarcomaClin Nucl Med 2016 41(3):235-36. [Google Scholar]
[12]. Yang WC, Yao M, Chen YH, Kuo SH, Complete response of myeloid sarcoma with cardiac involvement to radiotherapyJ Thorac Dis 2016 8(6):1323-28. [Google Scholar]
[13]. Dorfel D, Hantschel M, Federmann B, Haen S, Fend F, Muller II, Cardiac myeloid sarcoma: multimodality radiologic imaging features and pathologic correlationAm J Med 2016 129(8):e117-20. [Google Scholar]
[14]. Schaffer LR, Caltharp SA, Milla SS, Kogon BF, Cundiff CA, Dalal A, Rare presentation of four primary pediatric cardiac tumorsCardiovasc Pathol 2016 25(1):72-77. [Google Scholar]
[15]. Kim JG, Moon D, Yi JE, Youn HJ, Kim DW, Park GS, Recurrent cardiac chloroma presenting as acute chest painQJM 2014 107(5):381-82. [Google Scholar]
[16]. Mawad R, Wu D, Abkowitz JL, Walter RB, Myeloid sarcoma of the heartLeuk lymphoma 2012 53:2511-14. [Google Scholar]
[17]. Cash T, Becton D, Mian A, Cardiac myeloid sarcoma: a case report and review of literatureJ Pediatr Haematol Oncol 2011 33(7):e330-32. [Google Scholar]
[18]. Di Valentino M, Menafoglio A, Wyrttenbach R, Galliono A, An unusual cause of recurrent syncopeJ Am Coll Cardiol 2011 58(16):1728 [Google Scholar]
[19]. Makis W, Hickeson M, Derbekyan V, Myeloid sarcoma presenting as an anterior mediastinal mass invading the pericardium: Serial imaging with F-18 FDG PET/CTClin Nucl Med 2010 35(9):706-09. [Google Scholar]
[20]. Tirado CA, Chen W, Vadez F, Krandikar N, Arbini A, Acevedo I, Unusual presentation of myeloid sarcoma in a case of acute pyelomyelocytic leukaemia with a cryptic PML-RARA rearrangement involving multiple sites including the atriumCancer Gnet Cytogenet 2010 200(1):47-53. [Google Scholar]
[21]. Atallah A, Cheong BY, Bernicker E, Wilson JM, Cardiac chloroma: novel presentation and subsequent diagnosis with cardiac magnetic resonance imagingTex Heart Inst J 2010 37(2):242-43. [Google Scholar]
[22]. Tsai J, Lee EY, MDCT imaging findings of extramedullary granulocytic sarcoma of the heartJ Thorac Imaging 2010 25(1):W14-16. [Google Scholar]
[23]. Matkowskyj KA, Wiseman WR, Robin JC, Norvell JP, Puthumana J, Nelson B, Therapy related myelodysplastic syndrome presenting as fulminant heart failure secondary to myeloid sarcomaJ Haematop 2010 3(1):41-46. [Google Scholar]
[24]. Mignano JE, Chan MD, Rosenwald IB, Kimmelstiel CD, Wolfe LC, Intracradiac chloromaJ Pediatr Haematol Oncol 2009 31(12):977-79. [Google Scholar]
[25]. Tsai MH, Yang CP, Chung HT, Shih LY, Acute myeloid leukaemia in a young girl presenting with mediastinal granulocytic sarcoma invading pericardium and causing superior vena cava syndromeJ Pediatr Haematol Oncol 2009 31(12):980-82. [Google Scholar]
[26]. Kozelj M, Zorman D, Mrevlje B, Cernelc P, Zver S, Cardiac granulocytic sarcoma diagnosed by intracardiac echocardiography guided biopsyInt J Haematol 2008 88(1):101-03. [Google Scholar]
[27]. Rigamonti F, Beris P, Sanchez Pareja A, Meyer P, Ashrafpoor G, Atypical presentation of acute myeloid leukaemia: cardiac myeloid sarcomaInt J Haematol 2009 89(5):693-98. [Google Scholar]
[28]. Antic D, Vuckovic M, Elezovic I, Right atrial myeloid sarcoma causing superior vena cava syndromeBr J haematol 2008 141(2):134 [Google Scholar]
[29]. Diab M, Coloe J, Bechtel M, Extramedullary granulocytic sarcoma of the skin, mediastinum and pericardiumInt J Dermatol 2008 47(3):256-58. [Google Scholar]
[30]. Nasilowska-Adamska B, Majewski M, Seferynska I, Szczepinski A, Tomaszewska A, ProchrocecSobeiszek M, Predictive value of RT-PCR PML-RARA transcript monitoring for extramedullary relapse of acute promyelocytic leulemia in the pleura, heart and pericardium after allogeneic SCTAnn Transplant 2007 12(3):33-38. [Google Scholar]
[31]. Anton E, Cardiovascular involvement in adult granulocytic sarcomaAm J Haematol 2006 81(5):382-83. [Google Scholar]
[32]. Marcos-Alberca P, Ibáñez B, Rey M, Román A, Rábago R, Orejas M, Cardiac granulocytic sarcoma (chloroma): in vivo diagnosis with transesophageal echocardiographyJ Am Soc Echocardiogr 2004 17(9):1000-02. [Google Scholar]
[33]. Makaryus AN, Tung F, Liu W, Mangion J, Kort S, Extensive neoplastic cardiac infiltration in a patient with acute myelogenous leukaemia: role of echocardiographyEchocardiography 2003 20(6):539-44. [Google Scholar]
[34]. Erdol C, Ovali E, Baykan M, Granulocytic sarcoma presenting as a right atrial massActa Cardiol 2003 58(2):155-58. [Google Scholar]
[35]. Tillawi IS, Variakojis ID, Refractory right ventricular failure due to granulocytic sarcomaArch Pathol Lab Med 1990 114(9):983-85. [Google Scholar]
[36]. Foucar K, Foucar E, Willman C, Horvath A, Gerety RL, Nonleukaemic granulocytic sarcoma of the heart: A report of a fatal caseAm J Haematol 1987 25(3):325-32. [Google Scholar]
[37]. Lam KY, Dickens P, Chan AC, Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsiesArch Pathol Lab Med 1993 117(10):1027-31. [Google Scholar]
[38]. Tabbane C, Chadly A, Corcos V, Heldt A case of chloroma with cardiac metastasis in an 8-year-old childTunis Med 1960 38:153-64. [Google Scholar]
[39]. Sahu KK, Dhibar DP, Malhotra P, Isolated myeloid sarcomaOrbit 2016 35(6):351 [Google Scholar]
[40]. Sahu KK, Gautam A, Ailawadhi S, Re: FDG PET/CT findings of intracardiac myeloid sarcomaClin Nucl Med 2017 Mar 42(3):242-45. [Google Scholar]