Cancer affects all the communities around the world, approximately 10 million people are diagnosed with cancer and more than six million die of the disease every year. The most frequent cancers in Indian male are mouth, oropharynx, oesophagus and stomach [1].
Easy availability and wide usage of tobacco in India is posing serious concern in causing upper digestive and respiratory tract cancers. It has been estimated that almost 91% of oral cancers have a direct correlation with usage of tobacco. Cancers associated with the use of tobacco constitute for about 44.6% in men and 20% in women [2].
Studies have shown that when diagnosed 16 to 62% of oral cancer cases are associated with precancerous lesions and about 80% of them were preceded by oral potentially malignant disorders [3].
The end moieties of carbohydrate chains are sialic acids which have biological importance and are essential for the function of glycoconjugates, and they have been reported to be altered in cancer. A positive correlation between serum levels of TSA and LSA has been observed. Serum protein changes at the terminal end of α-2-6 sialic acid have been correlated with alterations in the levels of various forms of sialic acid and sialyltransferase. Malignancy cases have shown an increase in the levels of sialic acid and sialyltransferase when compared to healthy controls [4].
Majority of studies have been done in the past on systemic, head and neck cancers. Only very few studies have been done on oral potentially malignant disorders like oral submucous fibrosis and OL [5,6]. Therefore, an attempt has been made to study the levels of sialic acid in patients, exclusively with OL which was confirmed clinically and histopathologically.
The aim of this study was to evaluate TSA and LSA levels in serum in Leukoplakia with various histopathological grades and healthy controls.
Materials and Methods
The present cross-sectional study was conducted at Meenakshi Ammal Dental College and Hospital, Chennai for a period of two years.
Thirty normal, apparently healthy controls and thirty patients with OL, which were clinically diagnosed and further confirmed with histopathological examination, were included in the study. The study was approved by the Institutional Ethical Committee, and subjects gave their informed consent before the start of the study.
Inclusion and exclusion criteria: Subjects diagnosed of having OL and healthy controls in the age group of 18 to 60 years were included in the study while healthy individuals with past or present history of using tobacco or habits of areca nut chewing and or alcohol consumption, subjects under corticosteroid medication, history of systemic diseases and disorders altering the levels of TSA and LSA like Type II Diabetes mellitus, chronic obstructive pulmonary disease, cardiovascular disease, periodontal disease, acquired immunodeficiency syndrome, blood dyscrasias and conditions like pregnancy were excluded from the study [7–10].
Incisional biopsy was done and the samples were graded histopathologically into mild, moderate and severe dysplasia based on the criteria described by Warnakulasuriya S et al. [11].
Sample collection: Blood was collected by vein puncture using a syringe between 9:00 and 11:00 hours on every occasion to avoid any possible diurnal variation. The samples were centrifuged at 3000 rpm for about 15 minutes and the sera were separated from clotted blood which was then transferred to fresh containers to avoid any contamination. The separated serum was stored at -80°C until analysed spectrophotometrically.
Biochemical assay: TSA was determined using a periodiate-thiobarbituric acid method suggested by Lorant Skoza and Steven Mohos [12].
Serum levels of LSA levels were measured as suggested by Lopez Saez JJ and Senra VA [13].
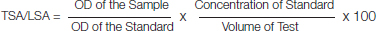
TSA/LSA means the concentration of TSA and LSA in serum that was calculated using the mentioned formula.
OD = Optical Density
Statistical Analysis
The data were analysed using the SPSS 16.0 version. The biochemical values were expressed as mean and standard deviation. Independent t-test was used to assess the TSA and LSA levels among OL and healthy controls. The LSA and TSA levels among different grades of OL was analysed using Kruskal Wallis and multiple comparison performed using Mann-Whitney U test.
Results
The descriptive data for TSA, LSA levels among OL and healthy controls is presented in [Table/Fig-1]. The mean TSA levels among those in the OL group (45.3±4.2) was significantly greater than healthy controls (29±2.2) when compared using independent t-test and similarly the mean LSA levels were higher in OL as compared to controls [Table/Fig-2].
Distribution and descriptive statistics of TSA (mg/dl) and LSA (mg/dl) in Oral Leukoplakia (OL) and Healthy controls.
| TSA | Mean (SD) | Range |
|---|
| ≤ 30 n (%) | 31.01-40.00 n (%) | ≥ 40.01 n (%) | | |
| OL | 0 | 5 (16.7) | 25 (83.3) | 45.3 (4.2) | 37.3-52 |
| Controls | 19 (63.3) | 11 (36.7) | 0 | 29 (2.2) | 21.75-32.57 |
| LSA | Mean (SD) | Range |
| ≤ 20 | >20 | | | |
| OL | 4 (13.3) | 26 (83.7) | | 23 (2.5) | 18.46-27.31 |
| Controls | 29 (96.7) | 1 (3.3) | | 16.74 (1.5) | 14.26-20.25 |
Mean and standard deviation of TSA and LSA level in Oral Leukoplakia (OL) and Healthy Controls.
| Group | Mean (SD) | T- value | p-value |
|---|
| TSA | OL | 45.31 (4.2) | 18.68 | <0.001* |
| Control | 29 (2.2) |
| LSA | OL | 23.02 (2.5) | 11.67 | <0.001* |
| Control | 16.74 (1.5) |
*p<0.05 is considered significant, Independent t-test
TSA levels of various grades of OL were compared using Kruskal Wallis test. There was a highly significant difference in the mean TSA levels in mild, moderate and severe OL (p<0.001). On multiple comparison the highest mean TSA levels was found in severe OL compared to moderate and mild OL respectively (p<0.05) [Table/Fig-3]. Similarly, LSA levels were compared to various grades of OL, but no significant difference was found among the mean values [Table/Fig-4].
Descriptive statistics of TSA level in accordance with grading.
| Descriptive statistics | No. Subjects | Mean | Standard deviation | Minimum | Maximum | F | p-valuea | Post-hocb |
|---|
| Mild | 20 | 43.13 | 3.32 | 37.26 | 48.27 | 18.215 | <0.001* | Severe*>Moderate*>Mild* |
| Moderate | 7 | 48.95 | 1.42 | 45.96 | 50.25 |
| Severe | 3 | 51.23 | 0.96 | 50.15 | 52.00 |
*p<0.05 is considered significant; a- Kruskal Wallis test
b- Multiple comparison performed using Mann-Whitney U Test.
Descriptive statistics of LSA level in accordance with grading.
| Descriptive statistics | No. Subjects | Mean | Standard deviation | Minimum | Maximum | Kruskal Wallis test | p-value |
|---|
| Mild | 20 | 22.47 | 2.66 | 18.46 | 27.00 | 4.94 | 0.085(NS) |
| Moderate | 7 | 24.85 | 1.53 | 22.00 | 26.66 |
| Severe | 3 | 24.36 | 2.64 | 22.22 | 27.31 |
NS = Not significant
Discussion
OL is a premalignant lesion and is defined as a predominantly white lesion of the oral mucosa that cannot be characterized as any other definable lesion [14]. It is one of the most common premalignant lesions with a strong male predilection. Its etiological factors are tobacco smoking, pan chewing, and alcohol consumption, viral agents such as Epstein - Barr virus, Human Papilloma virus, Candida Albicans and Nutritional deficiencies [3].
Dysplasia is a reversible entity hence it is considered to be a controlled cellular alteration. Therefore, when the underlying provocative stimulus is removed, the cellular dysplastic alterations go back to the normal [11,15].
Assessment of epithelial dysplasia may be subjective and there can be inter-observer variability that can exist in its interpretation. It is broadly divided into three categories such as mild, moderate and severe dysplasia [7].
Sialic acid, a negatively charged nine carbon monosaccharide is commonly present as n-acetyl neuraminic acid in the human body. It can exist as free, lipid bound in serum and as protein bound in saliva. The negatively charged sialic acid contributes to the cell to cell repulsion, cell to matrix interaction, cell to cell recognition and serves as a component of cell surface receptor. It is also present in body fluids like blood, plasma, breast milk, gallbladder excretion, synovial fluid, sweat, gastric juices and urine [16].
The incidence and death rate on account of malignancy have been showing multifold rise therefore, more efforts are required for the early diagnosis and timely treatment of this life threatening disease. Many researchers have been on the lookout for a specific, reliable and easily available biomarker, which can differentiate malignancy from premalignancy which in turn has high risk of developing cancer [17].
Off late importance of tumour markers have been noted in early detection and predicting the prognosis of precancerous lesions. In the last few decades, research efforts are being focussed on targeting membrane molecules in neoplastic transformation, particularly the cell surface glycoproteins which attributes for the malignant transformation of a cell. Among these glycol conjugates, sialic acid is present up to 30% in various glycoproteins [18–22].
The serum sialic acids in healthy individuals remain almost the same throughout their life and there can be a slight increase with advancing age. The likely reasons for the difference in values of serum sialic acid can be attributed to high carbohydrate content in diet, anaemia and hypoproteinemia prevailing in subcontinent [23].
Lipid bound sialic acid consists of glycolipid bound sialic acid only, wherein total sialic acid consists of both glycoprotein and glycolipid bound sialic acid. These glycoconjugates are released into the blood circulation either by secretion, increased turnover rate or by the shedding of malignant cells [3,5,24].
In the present study, the serum TSA and LSA levels between OL and healthy controls were compared and the results showed that there is a significant elevation in the serum levels of TSA and LSA. This is consistent with previous studies. [5,6,11,25,26]. [Table/Fig-4] represents LSA for epithelial dysplasia which is not significant.
It has been suggested that elevation in serum TSA can give an early indication of premalignant change. Sialic acid serves as an indicator for the changes noted in transformed cells. Biochemical changes in glycoproteins start at an early stage of tumourigenesis [26]. If routinely monitored, malignancy can be detected at an early stage [27].
Significant increase in serum TSA level with increasing grades of epithelial dysplasia was noted. No specific changes with LSA levels were noted in OL with increasing grades of epithelial dysplasia. Thus, based on the present study, it can be stated that in OL patients serum levels of TSA progressively increases with increasing grades of epithelial dysplasia and an increase in LSA is noted, when compared to healthy controls. This is consistent with the study done by Hemanth SC and Anand K and the reasons could be glycoconjugates have definite cut-off in precancer and healthy individuals. The elevation in the levels of these biomarkers, especially TSA, can give an early indication of a premalignant change [24,28].
The procedure of spectrophotometry includes the absorbency of a blank sample which does not contain a coloured compound wherein the sample contains a coloured compound. Spectrophotometer is used to evaluate coloured compounds in the visible range of light between 350-800 nm.
Spectrophotometer is one among the highly accurate instrument which is very sensitive and can therefore be precise to a greater extent, especially when it is required to determine the colour change. This method is also convenient for its use in the laboratorial experiments as it is inexpensive and relatively simple [29].
Irrespective of this study’s finding the potential benefits of sialic acid screening are enormous. The clinical diagnosis supplemented with sialic acid levels can gain diagnostic importance in near future. Moreover TSA and LSA levels can also be used as valuable aid in monitoring the prognosis of OL [27]. Since it is a pilot study with a relatively smaller sample size the results must be interpreted with caution. This limitation can be overcome by expanding to a larger sample size and also including other oral potentially malignant disorders in order to arrive at more definitive results.
Conclusion
In conclusion, serum TSA and LSA levels were increased in OL cases when compared to that of healthy controls. Further studies with large sample size are needed to be carried out to use serum as a prognostic indicator in screening, diagnosis, management and prevention of premalignant lesion such as OL.
*p<0.05 is considered significant, Independent t-test
*p<0.05 is considered significant; a- Kruskal Wallis test
b- Multiple comparison performed using Mann-Whitney U Test.
NS = Not significant