Micronucleus Assay in Urothelial Cells in Cancer Cervix
Suresh Kumar Sundararajan1, Pratheepa Sivasankari Natarajan2, Kanchana3
1 Assistant Professor, Department of Medical Oncology, Madras Medical College and Hospital, Chennai, Tamil Nadu, India.
2 Associate Professor, Department of Anatomy, SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu, India.
3 Professor, Department of Pathology, Madras Medical College and Hospital, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Suresh Kumar Sundararajan, C2, 402, Aksahya homes, Nandivaram, Guduvanchery, Chennai-603202, Tamil Nadu, India.
E-mail: drsure88@gmail.com
Introduction
Cancer ranks third among the ten leading global causes of death. To evaluate the genotoxic risks, observed as DNA damages, can be assessed by Micronucleus (MN) test.
Aim
To identify the occurrence of MN in normal and cancer cervix and find the correlation between MN and stage of cancer.
Materials and Methods
A total of 60 females were included in the study and visual examination of the cervix was done. Based on the examination two groups were formed: A- Normal cervix (n-23) and B- Presence of erosion or growth or ulcer etc., in cervix (n-37). Midstream urine sample was collected and centrifuged from the cases after getting the informed consent. Slides were prepared from the pellet, were fixed in methanol, glacial acetic acid fixative and stained with Giemsa and May Grunwald stain.
Statistical analysis was done by student’s t-test and chi-square test.
Results
A linear association was noted between the mean MN count and cancer cervix stage. Almost 18.2% of the Group A cases had significant MN count. Sensitivity and specificity of MN count in Group A was 83.8% and 82.6% respectively. The efficiency was 83.3%.
Conclusion
A statistically significant MN count was seen in the different stages of cancer cervix. There are cases who had normal findings on visual inspection of cervix but with significant MN count are prone for malignant transformation. MN assay is an easy, non-invasive, cost-effective method and can be used as a screening test for a large population.
Cervical malignancy, Genotoxicity, Nuclear abnormalities
Introduction
Cancer arises from one single cell. The transformation from a normal cell into a tumour cell is a multistage process, typically a progression from a precancerous lesion to malignant tumours. These changes are the result of the interaction between a person’s genetic factors and three categories of external agents, including: physical carcinogens, chemical carcinogens, and biological carcinogens, such as infections from certain viruses [1].
A data of cancer patients was compiled from 2004 to 2010 in India stated that the number of cancer cases had increased gradually with time [2].
The cancer cervix accounts for 12.60% amongst all cancers in Chennai, India and is the second most common cancer next to breast cancer [3].
MN are expressed in dividing cells that either contain chromosome breaks lacking centromeres (acentric fragments) and/or whole chromosomes that are unable to travel to the spindle poles during mitosis. At telophase, a nuclear envelope forms around the lagging chromosomes and fragments, which then uncoil and gradually assume the morphology of an interphase nucleus with the exception that they are smaller than the main nuclei in the cell, hence the term “Micronucleus” [Table/Fig-1]. MN, therefore, provide a convenient and reliable index of both chromosome breakage and chromosome loss.
Micronucleus- The arrow shows MN (Micronucleus – present inside the cytoplasm with the main nucleus sharing the same colour and 1/3rd size of the main nucleus).
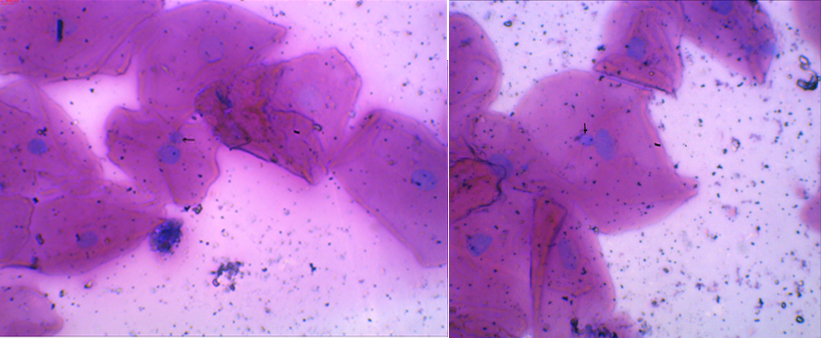
Scoring of MN can be performed easily and on different cell types relevant for human biomonitoring: lymphocytes, fibroblasts and exfoliated epithelial cells, without any additional in vitro cultivation step. MN observed in exfoliated cells is not induced when the cells are at the epithelial surface, but when they are in the basal layer [4].
The aim of the study was to identify the occurrence of MN in cases with normal cervix, cases with growth in the cervix and to identify the occurrence of MN in different stages of malignancy.
By using this technique cancer can be detected at an early stage.
Materials and Methods
The present descriptive study was conducted between the period of April 2009-2010. A total number of 60 patients with the complaints of leucorrhoea, postcoital bleeding, lower abdominal pain, intermenstrual bleeding and prolongation of menstrual bleeding, in the gynaecology Outpatient department at the Institute of Obstetrics and Gynaecology, Egmore, Tamil Nadu, India, were included in our study. They were grouped into two:
Patients whose visual inspection of cervix was normal were taken as Group A;
Patients whose visual inspection of cervix showed positive findings like erosion cervix, hypertrophied cervix, abnormal growth, ulcer or vasculature were taken as Group B.
Exclusion criteria were as follows:
Patients suffering from other malignancies.
Chronic alcoholics and smokers.
After getting the Institutional Ethical Committee acceptance, a proforma was prepared in order to record the history, general examination and pelvic examination. The slides were prepared by the protocol followed by Gandhi G et al., [5]. After obtaining the consent, the patient was requested to collect midstream urine sample (10 ml) in a sterile plastic container (sample to be taken at any time and no medications were recommended before the sample collection) and was transported to the laboratory. It was processed within three to four hours of sample collection. The sample was rinsed thrice in Phosphate Buffered Saline (PBS) with alternate centrifugations at 1200 rpm for 10 minutes. From the pellet smear preparations were made on precleaned slides. Up to two to three slides were made and were allowed to air dry. After air drying, the slides were kept in absolute methanol for 20 minutes for the fixation of cells. The slides were kept in May Grunwald stain for five minutes and were rinsed twice with distilled water. After washing, it was counter stained with giemsa stain for 8 to 10 minutes, followed by washing with distilled water. Stained slides were mounted with cover slip. The presence of MN was confirmed under oil immersion (100 X), observations were recorded and tabulated. Five hundred cells were recorded in each patient from the slides prepared and the incidence of micronucleus was recorded and the collected data was subjected to student’s independent t-test and chi-square test.
Statistical Ayalysis
The incidence of MN was recorded and the collected data was subjected to student’s independent t-test and chi-square test.
Results
The observed data was tabulated for analysis. Out of the 60 cases, 23 patients were in Group A (had no findings on visual inspection of cervix), 29 patients with growth in cervix and eight with erosion cervix were in Group B.
The mean age in Group A was 36.5 years and in Group B was 44 years. The age-range of the patients varied from 18- 65 years. Significant damage was observed only in patients who were 40-49 years in Group A and more than 50 years of age in Group B [Table/Fig-2]. A 50% of the cases in Group A were in the age group between 40-49 and had more than four MN, in Group B 38.7 % of the cases, above the age of 50 years had more than four MN [Table/Fig-3]. Among the 37 cases, 29 had growth on visual inspection of cervix and the rest seven had erosion cervix. All the 29 cases and the one case with erosion cervix were diagnosed as cancer cervix and the staging was done for the total 30 cases. According to the histopathological staging of the 30 cases in Group B were distributed as: one in stage I A, four in stage I B, eight in stage II A, six in stage II B, five in stage III A and six in stage III B. Among the Group B cases, stage II A had the highest MN Frequency and it was 22. 9% followed by stage II B and III B. Frequency of MN in each stage was calculated. The percentage represents the prevalence of MN in each stage [Table/Fig-4]. The MN count in Group B was statistically significant when compared to group A. There was a linear association between mean MN count and stage of cancer cervix and it was statistically significant. The sensitivity of the MN test in Group A was 83.8% and specificity was 82.6%. The efficiency of the test was found to be 83.3% [Table/Fig-5].
Mean age in Group A and Group B.
| Group | N | Mean | Std.deviation | Median | Minimum | Maximum |
|---|
| A | 23 | 36.59 | 12.912 | 39.00 | 18 | 65 |
| B | 37 | 44.84 | 11.934 | 43.00 | 18 | 72 |
| TOTAL | 60 | 41.76 | 12.843 | 40.00 | 18 | 72 |
Frequency of MN count in different age groups.
| Group | Age (years) | MN count |
|---|
| 3 | 4 & more |
|---|
| A | < 30 | 44.4 % | 25 % |
| 30-39 | 11.1 % | 0 |
| 40-49 | 38.9 % | 50% |
| >50 | 5.6 % | 25 % |
| B | <30 | 16. 7% | 9.7 % |
| 30-39 | 50% | 22.6 % |
| 40-49 | 33.3 % | 29 % |
| >50 | 0 | 38. 7 % |
Distribution of cases and MN frequency according to the staging of cancer cervix.
| Stage | N | Percent | MN Frequency |
|---|
| I A | 1 | 3.4 | 2.9% |
| I B | 4 | 13.6 | 11.4% |
| II A | 8 | 27.2 | 22.9% |
| II B | 6 | 20.4 | 17.1% |
| III A | 5 | 17.0 | 14. 3% |
| III B | 6 | 20.4 | 17. 1% |
| TOTAL | 30 | 100 | |
Distribution of cases with significant MN count and absent MN in urothelial cells in Group A and Group B.
| MN assay in urothelial cells | Test results(No. of individuals) | Total |
|---|
| Group A | Group B |
|---|
| MN Present | 4 | 31 | 35 |
| MN Absent | 19 | 6 | 25 |
| Total | 23 | 37 | 60 |
Sensitivity – 31/ 37 = 83.8%, Specificity – 19/ 23 = 82.6%, Efficiency - 50/ 60 = 83. 3%
Discussion
Cancer of breast and cervix are more prevalent in Indian females and accounts for 41.3%. Cancer cervix burden is alarmingly increasing and the projection would rise from 0.096 million to 0.148 million cases during 2011-2016 [6]. Genomic instability refers to an increased tendency of alterations in the genome during the life cycle of cells. It is a major driving force for tumorigenesis [7]. Genomic instabilities can be assessed by MN assay. MN originate from chromosome fragments, complete chromosomes lagging behind in anaphase or amplified genome regions, which are excluded from the nucleus by a process called nuclear budding [4].
Aging is the important factor which induces the formation of the important nuclear abnormality known as micronucleus [8], was an additive factor for the increased MN frequency in Group B cases above the age of 50 years. A progressive rise was seen in the frequency of MN with increasing age and maximum frequency was observed in older women beyond 40 years of age [5,8,9]. An increased frequency of chromosomal damage was associated with increasing chromosome instability in aged people but on the contrary Orta T et al., stated that the increase in the frequency of micronuclei was first observed up to 50 years of age and this increase was followed by a decrease with a further increasing age [8].
Significantly elevated frequency of Micronucleated (MNd) cells in all the patients irrespective of the stage of cancer was seen and the same was stated by Gandhi G et al., 2003 [10]. As per our observations the number of MN was significantly higher in stages II A followed by II B and III B above 50 years of age where the carcinoma in situ is formed or invades the neighbouring tissues [10–12].
The chromosomal abnormality caused by the environmental pollution might be the cause for the significant MN frequency (more than 4) seen in Group A [13]. MN frequency and staging of cancer cervix was statistically significant compared to control group. Genetic damage in cervix cancer patients was greater as compared to that in the control group [14].
There was a stepwise gradual increase in MN score from inflammatory to Atypical Squamous Cell of Undermined Significance (ASC-US) to Low-grade Squamous Intra-epithelial Lesion (LSIL) to High-grade Squamous Intra-epithelial lesion (HSIL), followed by a slight increase in Invasive Cancer (IC). The mean MN score was most significant in the HSIL and invasive group [15,16]. These results were well correlated with our results. MN frequency in our study was significantly higher in women with cervical cancer than in woman showing precursor lesions and this finding did not differ between the various stages of cancer [17,18].
Significant MN frequency was seen in four cases of Group A who had normal PAP smear. This is because the sensitivity of MN assay in detecting the genomic instability at an early stage in the carcinogenesis. The efficiency of the MN assay was found to be 83.3%, showed that the MN assay in urothelial cells has got a good negative predictive value. This study indicate that the MN assay in urothelial Cells, may prove beneficial to perform in screening programmes of cervix cancer, given the non-invasive method of sample collection, easier availability of urine and the rapidity of the assay.
Since the MN count was done manually, we could evaluate only 500 cells. This assay can be done as a screening test among the women in perimenopausal age group in large population by using automated MN assay. MN scoring can be interfered by the bacteria. Bacteria can be differentiated from MN by their characteristic shape, smaller size, colour, staining intensity. Females in the peri menopausal age group were not ready to volunteer for the cervical screening programmes or failed to come for the follow up. So, the early detection of the cancer cervix will be delayed. Large urine sample and contaminated urine sample with blood and bacteria are the major limitations of the study.
In India, as a secondary prevention, district cancer control programme project, in selected districts medical and paramedical staff of the district hospital and Anganwadi workers have been trained on the visual examination of the cervix, collection of PAP smears and referring the suspected cases to the district hospital for further evaluation [19].
Urine sample collection is much easier than collection of blood samples or cervix scrapings. The test can find applications in pilot screening programmes. In India, an inexpensive easy method like MN assay can be suggested as a part of routine gynaecological examination. The early detection and appropriate treatment measures can eventually assist in bringing down the morbidity and mortality.
Conclusion
A statistically significant MN count was seen above 50 years of age. A statistically significant MN count was seen in the different stages of cancer cervix. A linear association was noted between the mean MN count and cancer cervix stage. The cases with significant MN count with normal cervix, are more prone for malignant transformation. Those cases need to be followed up by doing cancer cervix screening once in a year.
Sensitivity – 31/ 37 = 83.8%, Specificity – 19/ 23 = 82.6%, Efficiency - 50/ 60 = 83. 3%
[1]. American cancer society [Internet]. American cancer society. 2014. Available from: http://www.cancer.org/acs/groups/cid/documents/webcontent/002048-pdf.pdf [Google Scholar]
[2]. Ali I, Wani AW, Saleem K, Cancer scenario in India with future perspectivesCancer Therapy 2011 8:56-70. [Google Scholar]
[3]. Leading Sites of Cancer [Internet]. Chapter 2. Three-Year Report of the PBCRs: 2012-2014. Available from: http://ncrpindia.org/Annual_Reports.aspx [Google Scholar]
[4]. Fenech M, The in vitro micronucleus techniqueMutation Research 2000 455:81-95. [Google Scholar]
[5]. Gandhi G, Sharma P, Kaur A, Badaruddoza The micronucleus test in urothelial cells and uterine smears of cervix cancer patients: a comparisonInt J Hum Genet 2003 3(2):121-26. [Google Scholar]
[6]. Dsouza N DR, Murthy NS, Aras RY, Projection of Cancer Incident Cases for India-Till 2026Asian Pacific Journal of Cancer Prevention [Internet] 2013 14(7):4379-86.Available from: https://www.researchgate.net/publication/264143909 [Google Scholar]
[7]. Shen Z, Genomic instability and cancer: An introductionJournal of Molecular Cell Biology 2011 3:1-3. [Google Scholar]
[8]. Orta T, Günebakan S, The effect of aging on micronuclei frequency and proliferation in human peripheral blood lymphocytesIndian J Hum Genet 2012 18(1):95-100. [Google Scholar]
[9]. Misra JS, Srivastava S, Singh U, Srivastava AN, Risk-factors and strategies for control of carcinoma cervix in India: Hospital based cytological screening experience of 35 yearsIndian Journal of Cancer 2009 46(2):155-59. [Google Scholar]
[10]. Gandhi G, Kaur B, Elevated frequency of micronuclei in uterine smears of cervix cancer patientsCaryologia 2003 56(2):217-22. [Google Scholar]
[11]. Jayasabarinathan M, Kaur S, Vijayadevi S, Micronucleus assay in urothelial cells is a diagnostic indicator in cancer cervixJournal of Evolution of Medical and Dental Sciences [JEMDS] 2015 4(30):5142-49. [Google Scholar]
[12]. Navya BN, Supriya SP, Doppa G, Alva SR, Micronucleus test as a prognostic marker in cervical lesions on pap smearSch J App Med Sci [Internet] 2016 4(10):3758-63.Available from: http://saspublisher.com/sjams/ [Google Scholar]
[13]. Klumpp A, Ansel W, Klumpp G, Calatayud V, Pierre J, He GS, Tradescantia micronucleus test indicates genotoxic potential of traffic emissions in European citiesEnvironmental Pollution 2006 139:515-22. [Google Scholar]
[14]. Gayathri BN, Kalyani R, Hemalatha A, Vasavi B, Significance of micronucleus in cervical intraepithelial lesions and carcinomaJ Cytol 2012 29(4):236-40. [Google Scholar]
[15]. Hua shi Y, Wei Wang B, Tuokan T, Zhi Li Q, Jing Zhang Y, Association between micronucleus frequency and cervical intraepithelial neoplasia grade in thin prep cytological test and its significanceInt J Clin Exp Pathol 2015 8(7):8426-32. [Google Scholar]
[16]. Bueno CT, Dornelles da Silva CM, Barcellos RB, Silva Jda, dos Santos CR, Menezes JES, Association between cervical lesion grade and micronucleus frequency in the Papanicolaou testGenetics and Molecular Biology 2014 37(3):496-99. [Google Scholar]
[17]. Ambroise MM, Kanchana B, Manjiri P, Predictive value of micronucleus count in cervical intra epithelial neoplasia and carcinomaTurk Patolji Derg 2013 29:171-78. [Google Scholar]
[18]. Aires GMA, Meireles JRC, Olivera PC, Oliveira JL, Araujo EL, Pires BC, Micronuclei as biomarkers for evaluating the risk of malignant transformation in the uterine cervixGenetics and Molecular Research 2011 10(3):1558-64. [Google Scholar]
[19]. Murthy NS, Mathew A, Cancer epidemiology, prevention and controlCurrent Science 2004 86(4):518-27. [Google Scholar]