The success of endodontic therapy is dependent on the maintenance of aseptic chain right from access opening to permanent coronal restoration of the tooth. The practitioner must be concerned not only with endogenous oral microbial flora, but also with exogenous bacterial contamination as well [1]. For optimum infection control, every instrument and material placed in the root canals should be sterile [2]. This holds true for obturating materials also. GP cones are the most commonly used core material for the obturation of root canal system [3]. Although GP cones are manufactured under aseptic conditions and present potential antimicrobial properties especially owing to zinc oxide component [4], they can be contaminated by aerosols, improper storage and physical handling of the cones [2].
It would be worthwhile if obturation material used to fill the root canal system were free from pathogenic microorganisms, because endodontic therapy is mainly a procedure of decontamination in order to prevent the dissemination of microorganisms throughout the root canal system and periapical tissues [9]. Hence, rapid chair side disinfection of GP cones is of utmost importance to maintain aseptic chain during root canal treatment [10]. As GP cones are heat labile, moist and dry heat sterilization cannot be used as it causes alteration of GP structure.
Hence, cold sterilization using chemical disinfectants such as ethyl alcohol, paraformaldehyde, sodium hypochlorite and formocresol are routinely used, which usually takes 1-25 minutes for disinfection. Amongst all, sodium hypochlorite is most effective as it takes one minute time for disinfection. However in all concentrations, crystal deposition on the surface of GP cones is reported, hampering the bond of the sealers with GP cones, leading to microleakage [10].
Herbal solutions which are commonly available in Indian market have shown dramatic results as antimicrobials, anticancer, antidiabetic, immune modulatory, in respiratory diseases, in liver disorders and as cosmetics agents [11–13]. The popularity of herbal medications has increased due to the search for cheaper, more accessible and natural form of alternatives [10]. However, data regarding their use as disinfection agents in endodontic practice is still lacking. Thus, herbal solutions like Aloevera Juice, Amla Juice, Pancha Tulsi were used in the present study.
Therefore, the purpose of the present study was to compare the efficacy of Aloevera Juice, Amla Juice and Pancha Tulsi in disinfecting GP cones.
Materials and Methods
The present in vitro study was conducted at Averin Biotech Private Limited, Hyderabad, Telangana State, India. Ninety GP cones (2% taper, Size 80, Dentsply) which were taken from freshly opened boxes under sterile conditions were included in the study. Damaged and bent cones were discarded.
Microbial suspension of E. faecalis (ATCC2912) and S. aureus (ATCC6538) of approximately 108 CFU/ml in Trypticase Soy Broth (TSB) (HiMedia Laboratories) were the test organisms that were used.
The herbal solutions used for the study were Aloevera Juice (The Unati Co-op., Marketing-Cum Processing Ltd., India), Amla Juice (The Unati Co-op., Marketing-Cum Processing Ltd., India) and Pancha Tulsi (Deltas Pharma, India).
Artificial Contamination of GP Cones: Eighty GP cones were divided into two groups: Group A and Group B with 40 cones in each group. Group A with 40 cones were contaminated with 20 ml of microbial suspension of S. aureus for 30 minutes. Group B with 40 cones were contaminated with 20 ml of microbial suspension of E. faecalis for 30 minutes.
Disinfection of GP Cones: After artificial contamination, GP cones were immersed in the respective disinfectant solutions for one minute. Based on the disinfectant used, GP cones from both the groups were subdivided into four groups with 10 cones in each group.
Group I A: Ten contaminated cones (S. aureus) immersed in Aloevera Juice.
Group II A: Ten contaminated cones (S. aureus) immersed in Amla Juice.
Group III A: Ten contaminated cones (S. aureus) immersed in Pancha Tulsi.
Group IV A: Ten contaminated cones (S. aureus) without any disinfectant and served as positive control.
Group I B: Ten contaminated cones (E. faecalis) immersed in Aloevera Juice.
Group II B: Ten contaminated cones (E. faecalis) immersed in Amla Juice.
Group III B: Ten contaminated cones (E. faecalis) immersed in Pancha Tulsi.
Group IV B: Ten contaminated cones (E. faecalis) without any disinfectant and served as positive control.
Group V Consisted of 10 uncontaminated cones which served as negative control.
All the cones were individually transferred to sterile test tubes containing 10 ml of thioglycollate media (HiMedia Laboratories) and incubated at 37°C for seven days. After seven days, a micropipette was used to transfer the thioglycollate media to a petridish containing Brain Heart Infusion (BHI) agar. A sterile cotton tip was used to spread the thioglycollate media in a thin layer over BHI agar. The plates were then incubated for 48 hours aerobically at 37°C and the Colony Forming Units (CFU) were counted with digital colony counter.
Statistical Analysis
The values obtained were statistically analyzed using computer software Statistical Package for Social Sciences (SPSS) version 17.0. One way analysis of variance (ANOVA) followed by Tukey Post-hoc test was used to analyze the data. The level of significance was set at p<0.01. A p-value <0.01 indicates that the values are 99% accurate.
Results
The results of this study are shown in [Table/Fig-1,2]. There was a statistically significant difference (p<0.01) between the test groups in the mean colony forming units.
Comparison of the mean number of colonies (X 10-1) among the disinfecting solutions against Staphylococcus aureus using ANOVA and Post-hoc tests.
| Organism | Group | n | Mean | SD | Median | Min. | Max. | ANOVA | Post-hoc |
|---|
| Staphylococcus aureus | Aloevera Juice (I A) | 10 | 100.60 | 11.2 | 93.50 | 90 | 115 | F=386.6p<0.01* | I A>II A, III A, V; <IV A |
| Amla Juice(II A) | 10 | 67.60 | 4.30 | 68.00 | 61 | 75 | II A>III A, V; <I A, IV A |
| Pancha Tulsi (III A) | 10 | 2.20 | 3.46 | 0.000 | 0 | 10 | III A>V; <I A, II A, IV A |
| Positive Control (IV A) | 10 | 142.90 | 145.0 | 21.0 | 99 | 170 | IV A>I A, II A, III A, V |
| Negative Control (V) | 10 | 1.60 | 1.90 | 1.000 | 0 | 5 | V<I A, II A, III A, IV A |
*indicates statistically significant difference between the experimental groups
Comparison of the mean number of colonies (X 10-1) among the disinfecting solutions against Enterococcus faecalis using ANOVA and Post-hoc tests.
| Organism | Group | n | Mean | SD | Median | Min. | Max. | ANOVA | Post-hoc |
|---|
| Enterococcus faecalis | Aloevera Juice (I B) | 10 | 110.10 | 2.96 | 110.0 | 106 | 115 | F=386.6p<0.01* | I B>II B, III B, V;<IV B |
| Amla Juice (II B) | 10 | 69.000 | 3.68 | 69.50 | 63 | 75 | II B>III B, V;<I B, IV B |
| Pancha Tulsi (III B) | 10 | 2.8000 | 4.92 | 0.000 | 0 | 14 | III B>V; <I B, II B, IV B |
| Positive Control (IV B) | 10 | 154.30 | 23.0 | 156.0 | 110 | 190 | IV B>I B, II B, III B, V |
| Negative Control (V) | 10 | 1.6000 | 1.90 | 1.000 | 0 | 5 | V<I B, II B, III B, IV B |
*indicates statistically significant difference between the experimental groups
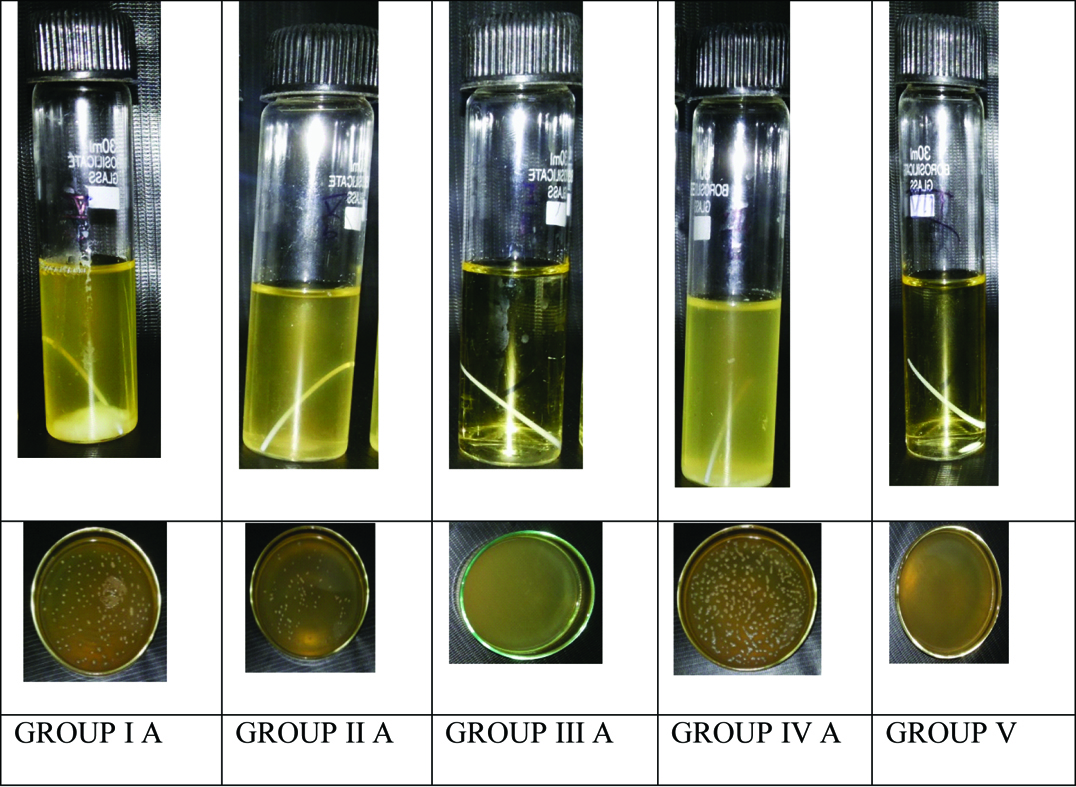
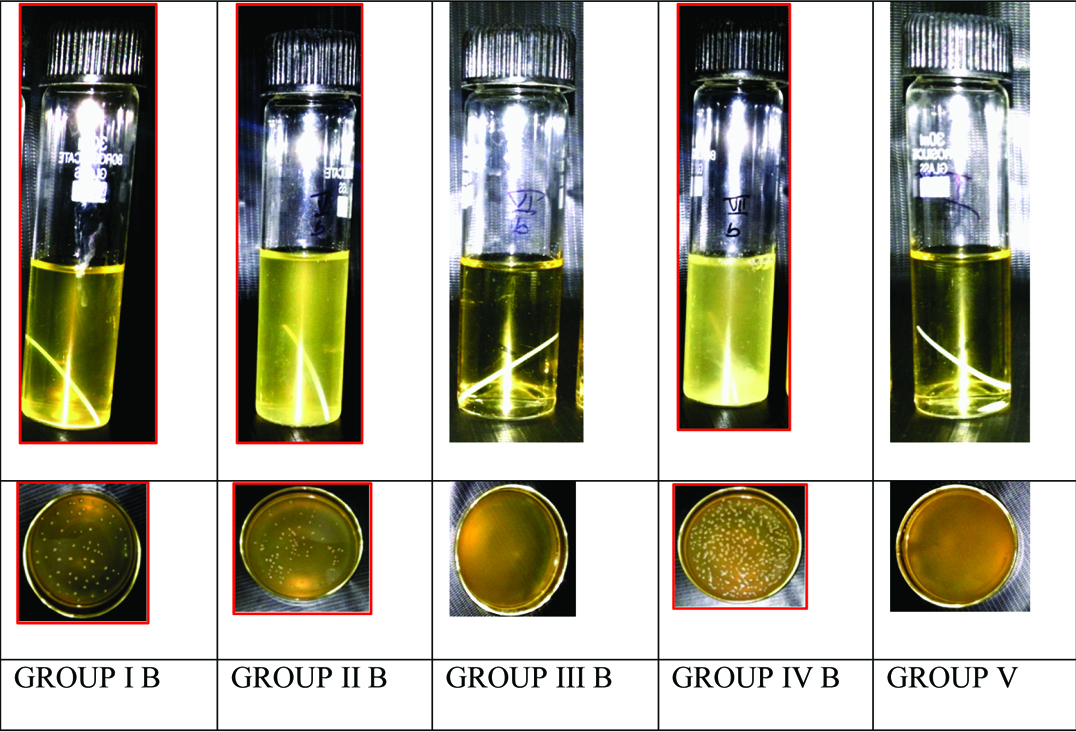
Among the experimental groups, Pancha Tulsi exhibited least number of colonies when compared to other disinfecting solutions. Amla Juice was found to be the second most effective disinfecting solution while Aloevera Juice was least effective among the solutions tested. Turbidity among the disinfecting solutions in Group A (S.aureus) and Group B (E. faecalis) are illustrated in [Table/Fig-3,4] respectively.
Turbidity in Group A (S. aureus).
Turbidity in Group B (E. faecalis).
Discussion
The important step during the endodontic treatment is sterilization of endodontic instruments and materials [3]. GP cones have been selected as the material of choice for root canal obturation because of properties such as biocompatibility, radio-opacity, dimensionally stability, antibacterial activity and also easy removal from root canal [14]. Even though GP cones are produced under aseptic conditions, once exposed to the dental office environment or even by handling, they can be contaminated by variety of micro-organisms [15]. Studies have revealed the presence of micro-organisms in 5%-19% of freshly opened GP packs [1,6,15]. GP cones cannot be sterilized by the conventional process in which moist or dry heat is used because this may cause alteration to the GP structure due to their thermoplastic characteristics [3]. Therefore, a chair side decontamination using a chemical agent should be adopted in routine endodontic practice to render them, free of microorganisms [9,16,17]. Furthermore, it is difficult to know beforehand how many accessory cones will be used during lateral condensation. Therefore, an effective chemical agent that acts quickly against surface contaminant microorganisms should be used for their decontamination [6].
Among various methods, immersing in 5.25% NaOCl for one minute is a gold standard for rapid decontamination of GP [6,16], whereas, chlorhexidine was effective only at five minutes [2]. However, Short RD et al., in their SEM study showed that GP cones immersed in 5.25% and 2.5% NaOCl revealed that chloride crystal formation may interfere with bonding, thus compromising the obturation seal [15]. Pang NS et al., studied the surface changes and physical properties of GP cones after chemical disinfection with 2% chlorhexidine, 5.25% NaOCl and ChloraPrep. These disinfectants significantly increased their elongation rate compared to fresh GP cones, especially in the ChloraPrep [18]. Also, one minute treatment with 5.25% NaOCl increased the elasticity of GP cones [19].
In this study, efficacy of three herbal solutions against two bacteria was evaluated. E. faecalis was chosen in this study because of its great capacity to live for long periods without nutrients and its great adaptation to the endodontic system. Also, E. faecalis is the bacteria most frequently isolated in chronic and persistent post treatment infections [20]. Although, S. aureus are seldom found in the infected root canals despite the fact that they are normal inhabitants of saliva and human skin, GP cones can be easily contaminated during storage if incorrectly manipulated [6,21].
Athiban PP et al., proved the antimicrobial efficacy of Aloevera gel against three microbes by forming effective inhibition zones which was almost equal to 5.25% NaOCl [22]. Shenoi PR et al., evaluated the efficacy of three herbal gels in disinfecting GP cones. They concluded that all the gels showed inhibition zones nearly equal to 5.25% NaOCl [10]. Thus, herbal products are effective substitutes to the chemical disinfectants. Hence, the present study was taken up to verify the ability of three herbal solutions to disinfect GP in a stipulated time period of one minute.
The findings of the present study revealed that Pancha Tulsi showed better disinfection action when compared to Amla Juice and Aloevera Juice. Phytochemical analysis of Pancha Tulsi revealed the antimicrobial compounds like glycosides, tannins, alkaloids, anthra-quinones, saponins, resins, polysaccharides, steroidal terpens, cardiac glycosides, steroidal ring and flavonoids [23]. Presence of apparently more number of bioactive components would have contributed to the better disinfection action of Pancha Tulsi when compared to Amla Juice and Aloevera Juice.
The current study showed that Amla Juice was the second best disinfectant among the experimental groups. Presence of phytochemical components like tannins, terpenoids, alkaloids, flavonoids, various polyphenols, quercetin, gallic acid, pectin, and vitamin C would have contributed to better disinfection action [24]. Shoko T et al., confirmed that phenolics were the most important compounds against bacteria, among those, gallic acid was identified as the most active compound for inhibition of bacteria [25]. The inhibitory effect of phenolic compounds could be explained by adsorption to cell membranes, interaction with enzymes, substrate and metal ion deprivation [26].
The disinfection action of Aloevera Juice can be attributed to anthra-quinones, saponins, pyrocatechol, cinnamic acid, ascorbic acid, sterols, p-coumaric acid [22]. Though Aloevera Juice showed antimicrobial activity, the lesser disinfection action was due to lesser acidic content and lower amount of total monomeric anthocyanins when compared to the other test groups [26].
Clinical Implication
Although GP cones are usually supplied in aseptic packages, once opened and used, they may be contaminated. Hence, a supplementary disinfection of GP cones is essential to avoid canal recontamination.
Limitation
Root canal infections are polymicrobial in nature. However, this is only an antibacterial study.
Hence, further studies are required to evaluate the disinfection action against other microbes like fungi, viruses and yeasts. Also, future research regarding the exact mechanism of disinfection action of the herbal solutions is required.
Conclusion
Within the limitations of the study, all the tested herbal solutions demonstrated disinfection action against S. aureus and E. faecalis. There was a significant difference found in the number of colony forming units between experimental and control groups. Pancha Tulsi exhibited good disinfection action against S. aureus and E. faecalis, followed by Amla Juice. Among the experimental groups, Aloevera Juice exhibited least disinfection action against both the microbes.
*indicates statistically significant difference between the experimental groups
*indicates statistically significant difference between the experimental groups